1
Biologie Cellulaire de la Synapse, Ecole Normale Superieure, Paris, France
2
Center for Molecular Neurobiology, ZMNH, University of Hamburg Medical School, Hamburg, Germany
Regulation of synaptic transmission is essential to tune individual-to-network neuronal activity. One way to modulate synaptic strength is to regulate neurotransmitter receptor numbers at postsynaptic sites. This can be achieved either through plasma membrane insertion of receptors derived from intracellular vesicle pools, a process depending on active cytoskeleton transport, or through surface membrane removal via endocytosis. In parallel, lateral diffusion events along the plasma membrane allow the exchange of receptor molecules between synaptic and extrasynaptic compartments, contributing to synaptic strength regulation. In recent years, results obtained from several groups studying glycine receptor (GlyR) trafficking and dynamics shed light on the regulation of synaptic GlyR density. Here, we review (i) proteins and mechanisms involved in GlyR cytoskeletal transport, (ii) the diffusion dynamics of GlyR and of its scaffolding protein gephyrin that control receptor numbers, and its relationship with synaptic plasticity, and (iii) adaptative changes in GlyR diffusion in response to global activity modifications, as a homeostatic mechanism.
Glycine receptors (GlyRs) mediate synaptic inhibition in brain and spinal neurons and locate either at glycinergic (Triller et al., 1985
, 1987
; Betz, 1991
) or mixed glycinergic/GABAergic postsynaptic sites (Lévi et al., 1999
; Dumoulin et al., 2000
). GlyRs bind directly the scaffold protein gephyrin (Meyer et al., 1995
) at different cellular compartments. GlyR molecules are associated with gephyrin in intracellular vesicles (Hanus et al., 2004
), which apply gephyrin as a cargo adaptor and link the receptor to microtubule-dependent motor proteins that power long distance bidirectional transport between neuronal somata and distal neurites (Maas et al., 2006
, 2009
). In addition to its association with GlyR during intracellular transport, gephyrin stabilizes the receptor once inserted in the surface membrane, in particular at synaptic sites. The first evidence of a functional synaptic microdomain was the detection by light and electron microscopy of GlyR and gephyrin aggregates in front of the presynaptic bouton (Triller et al., 1985
). Synaptic gephyrin clustering precedes the postsynaptic localization of GlyRs in vivo as well as in vitro (Kirsch et al., 1993b
; Bechade et al., 1996
; Colin et al., 1998
; Dumoulin et al., 2000
). The recruitment of GlyR by gephyrin within clusters depends on a functional receptor (Kirsch and Betz, 1998
; Lévi et al., 1998
) and requires the presence of the appropriate presynaptic innervation (Lévi et al., 1999
). Disruption of the gephyrin scaffold by antisense oligonucleotides or after intracellular antibody capture prevents the formation of GlyR clusters (Kirsch et al., 1993a
; Zacchi et al., 2008
). The same consequence is observed in the gephyrin-deficient mouse (Feng et al., 1998
). Actually, gephyrin molecules are able to trimerize and dimerize simultaneously via its G- and E-domains, respectively (Sola et al., 2001
, 2004
; Saiyed et al., 2007
). It has been postulated that this arrangement leads to the formation of a hexagonal lattice in the postsynaptic density (Xiang et al., 2001
; Sola et al., 2004
), offering multiple binding sites for GlyRs and representing a structure for new gephyrin molecules to be added (see Figure 1
).
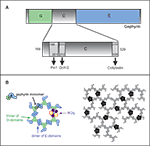
Figure 1. Gephyrin domains and structural organization. (A) Schematic depiction of the three gephyrin domains (G, C, E): the N-terminal G-domain (G) and the -terminal E-domain (E) are separated by a central C-domain (C). The C domain is magnified below. Sequences of the binding sites for Pin1 (Zita et al., 2007
), Dlc1/2 (Fuhrmann et al., 2002
) and collybistin (Kins et al., 2000
) are depicted by arrows. Numbers represent amino acid positions within the gephyrin protein. (B) The gephyrin «hexagonal lattice» oligomerization model: E and G-gephyrin domains are able to dimerize and trimerize, respectively (left panel). Combination of these two properties would lead to a hexagonal structure of gephyrin (right panel) underneath the postsynaptic membrane, where GlyR (in black) would anchor itself through the binding of the intracellular loop of the beta subunit with the E-domain of gephyrin.
Here we discuss active and passive parameters of GlyR and gephyrin dynamics at both intracellular and cell surface compartments. We particularly focus on whether and how changes in neuronal activity modulate these processes underlying the regulation of synaptic strength and/or plasticity.
KIF and Dynein-Related Transport Processes
Neurons are highly polarized cells with axons and dendrites. Many neuronal molecules are needed in one but not in the other compartment and require sorting and long-distance delivery into peripheral neurites (Hirokawa and Takemura, 2005
). Within axons and dendrites, longitudinally oriented microtubules serve as rails for ATP-dependent molecular motors, which convert chemical energy into mechanical work and mediate intracellular transport of membraneous organelles and macromolecular complexes (Desai and Mitchison, 1997
; Hirokawa and Takemura, 2005
; Caviston and Holzbaur, 2006
). Microtubules are unipolar structures, made of α- and β-tubulin subunits that lead to plus- and minus-ends within the polymer. In axons and distal dendrites, the fast growing plus-ends point away from the cell body (Baas et al., 1988
). Motor proteins of the kinesin (KIF) and dynein superfamilies drive molecular cargo along microtubule tracks. Most KIFs are plus end-directed motors and participate in anterograde transport that selectively guides molecules from the soma into neurite processes. In contrast, cytoplasmic dynein motors are minus end-directed and mediate retrograde transport from the periphery toward the cell body (Hirokawa and Takemura, 2005
; Caviston and Holzbaur, 2006
), in which cargo molecules eventually undergo degradation. In close proximity to the plasma membrane, actin microfilaments also serve as rails for local molecular transport. Here, unconventional myosins often mediate the final steps of plasma membrane delivery or the initial steps of surface membrane internalization, respectively (Bridgman, 2004
). In accordance with a distinct subcellular distribution of cytoskeletal elements, individual cargoes are thought to switch between actin- and microtubule-based transport (Radtke et al., 2006
) to travel toward submembrane or intracellular compartments.
Most synaptic proteins including the GlyR are synthesized in the cell body. They require active long-distance vesicle transport into neurites and toward the plasma membrane to reach their functional destination, the synapse compartment. Molecular motors mediate intracellular cargo transport with velocities in the range of μm/sec on average (Hirokawa and Takemura, 2005
) and live cell imaging identified mobile transport packets of GlyR fusion proteins that bidirectionally traveled through neurite processes (Maas et al., 2006
). However, under conditions of synaptic plasticity that require the rapid delivery of newly synthesized material, long-distance transport might be limited in providing sufficient amounts of synaptic components on a fast time scale. Alternatively, neurons use RNA-protein granules (Kanai et al., 2004
) to target individual messenger RNAs (mRNAs) into dendrites and apply local translation in close proximity to axo-dendritic contacts (Sutton and Schuman, 2006
). Consistently, GlyR α-subunit mRNAs were found to localize in neuronal dendrites (Racca et al., 1997
, 1998
; Gardiol et al., 1999
) and the GlyR binding protein gephyrin was shown to interact with RAFT1/mTOR (Sabatini et al., 1999
), a critical signalling component in translational control (Ma and Blenis, 2009
), suggesting that glycinergic synapses represent sites of local translation under certain conditions.
Originally, GlyRs (Bechade et al., 1996
) and the GlyR-interacting protein gephyrin (Seitanidou et al., 1992
; Colin et al., 1996
) were identified at intracellular sites in neurons, and depolymerization of microtubules dispersed the subcellular accumulation of both proteins (Kirsch and Betz, 1995
). A direct association of gephyrin with the light chains Dlc-1/Dlc-2, components of the microtubule-dependent dynein motor, suggested that microtubule transport might be involved in the subcellular localization of these factors (Fuhrmann et al., 2002
) (Figure 1
).
Neuronal coexpression of epitope-tagged gephyrin and GlyR α1 subunits confirmed that gephyrin indeed localizes to GlyR-containing intracellular vesicle structures (Hanus et al., 2004
). In fact, gephyrin accelerated the accumulation of GlyRs at the cell surface and depolymerization of microtubules interfered with these targeting processes (Hanus et al., 2004
). These data were complemented by functional evidence that active microtubule-dependent motor protein complexes interact, colocalize and comigrate with GlyR- and gephyrin-fusion proteins through neurite processes over time (Maas et al., 2006
, 2009
) (Figure 2
). For anterograde transport toward the plasma membrane, GlyR-gephyrin complexes were found to apply conventional kinesin (KIF5) as their driving force (Maas et al., 2009
). Specific blockade of KIF5’s motor function interfered with the delivery of gephyrin into peripheral neurites and the knockdown of gephyrin gene expression caused in turn a significant reduction in GlyR surface membrane delivery (Maas et al., 2009
). In contrast, GlyR-gephyrin transport toward the cell center is mediated by the retrograde-directed dynein motor complex (Maas et al., 2006
), known to participate in both receptor internalization processes downstream of the sorting endosome (Traer et al., 2007
) and long distance retrograde trafficking through neurite processes (Caviston and Holzbaur, 2006
). In general, mobile GFP-gephyrin transport packets in the synapse are continuously added to and removed from immobile postsynaptic gephyrin scaffolds in the minute range (Figure 2
) and rapidly switch between neighbouring synapses over time (Maas et al., 2006
). Notably and similarly to AMPA receptor (AMPAR) transport complexes (Setou et al., 2002
), the postsynaptic anchoring protein (gephyrin) was found to act as a cargo adaptor that directly links the receptor (GlyR) to either its kinesin or dynein motor, respectively (Kneussel, 2005
; Maas et al., 2006
, 2009
).
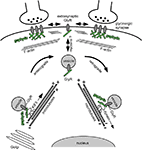
Figure 2. GlyR-gephyrin intracellular cytoskeleton transport. Newly synthesized glycine receptors (GlyRs) that leave the Golgi compartment reach the plasma membrane through active transport mechanisms along cytoskeletal elements. KIF5 motor proteins connect to vesicular GlyRs via gephyrin (green) that serves as a cargo adaptor in the transport complex. The KIF5/gephyrin/GlyR complex moves in anterograde directions toward the plus-ends of microtubules. It is currently unclear whether myosins mediate the final steps of GlyR surface membrane delivery and the initial steps of plasma membrane internalization, respectively, to traverse the submembrane actin cortex. At postsynaptic sites, gephyrin (green) forms a submembrane scaffold and mediates GlyR clustering. Exo-/ and endocytosis of receptors is thought to occur at extrasynaptic sites. Upon GlyR internalization, a GlyR/gephyrin/dynein transport complex mediates retrograde minus end-directed microtubule transport to intracellular compartments. Cytoplasmic dyneins are thought to participate in endocytic processes downstream on the sorting endosome (e.g. delivery to multivesicular bodies and/or lysososmes). In analogy to the anterograde GlyR transport complex, gephyrin (green) serves as a cargo adaptor that connects the vesicular receptor with its motor.
Together, independent approaches have revealed that GlyR and gephyrin functionally associate already at the intracellular level prior to their role in receptor scaffolding at postsynaptic sites. Although both proteins were found to undergo long-distance intracellular transport in a microtubule-dependent manner, it is currently unclear whether myosin-type motor proteins participate in local GlyR-gephyrin transport at actin-rich compartments underneath the neuronal plasma membrane (Figure 2
). GlyR-gephyrin intracellular transport in neurons resembles other receptor-motor systems heading to and from glutamatergic spine synapses (Kneussel, 2005
). However, which functional parameters regulate transport and drive GlyR-gephyrin-complexes particularly to inhibitory shaft synapses is currently barely understood.
Activity-Dependent Mechanisms Regulating Microtubule Structure
Functional regulation of active intracellular transport could occur at least at three different levels. First, neurons apply the alternate use of individual cargo adaptors, which connect motors with selected cargoes and are thought to mediate transport specificity (Setou et al., 2000
, 2002
; Hirokawa and Takemura, 2005
; Maas et al., 2006
). In addition, cargo adaptors participate in the regulation of the trafficking direction, for instance whether transport complexes selectively move into axons or dendrites (Setou et al., 2002
). Second, activity-dependent phosphorylation of motor proteins upon a Ca2+-dependent activation of the kinase CaMKII, has been shown to regulate synaptic microtubule transport (Guillaud et al., 2008
). In fact, phosphorylation of the KIF17 tail led to a local dissociation of an NMDA receptor motor-cargo complex, thereby releasing the cargo vesicle in close proximity to the synapse. Whether similar regulatory signals apply to all synaptic transport systems including the GlyR-gephyrin complex, requires further investigation. However, it is an attractive hypothesis to consider that a local slow-down or dissociation of intracellular trafficking complexes might increase the probability to exchange cargo between intracellular transport and surface membrane compartments. Notably, this model suggests that synaptic activation enables individual synapses to capture new molecules from a nearby flow of intracellular cargo.
A third way to regulate transport is to modify the structure of the tracks along which motors move. Different post-translational modifications (PTMs) of α- and β-tubulin have been described, which include phosphorylation, polyglutamylation, polyglycylation, tyrosination, methylation and acetylation (Verhey and Gaertig, 2007
). Upon these modifications, microtubules create diverse arrays with specific cellular functions in neurons. The addition of post-translational tubulin signals generates subpopulations of microtubules that selectively affect downstream microtubule-based functions, such as for instance the binding of various microtubule-associated proteins (MAPs) that could in turn affect kinesin motility (Fukushima et al., 2009
) (Figure 3
). Posttranslational addition of elongated polyglutamyl side chains to tubulin had been previously shown to functionally regulate the transport of synaptic vesicles, a cargo of KIF1A (Ikegami et al., 2007
). A recent study further revealed that tubulin polyglutamylation in neurons is significantly enhanced upon increased neuronal activity, induced through either AMPAR activation or blockade of the inhibitory GlyR with its antagonist strychnine (Maas et al., 2009
) (Figure 3
A). With respect to GlyR-gephyrin intracellular transport, increased tubulin polyglutamylation negatively interfered with gephyrin delivery into peripheral neurites and led to protein accumulation in the cell soma. Notably, these effects could be prevented upon gene expression knockdown or functional inactivation of the respective enzyme, known as neuronal polyglutamylase. This indicates that an activity-dependent signalling cascade crosstalks to enzymes involved in microtubule modification (Maas et al., 2009
). Polyglutamylation further regulates the binding of MAPs to microtubules (Bonnet et al., 2001
) and MAP2 is known to negatively influence kinesin transport (von Massow et al., 1989
; Lopez and Sheetz, 1993
). Consequently, strychnine blockade of GlyRs over 8h significantly increased MAP2 binding to microtubules and reduced KIF5C particle mobilities, the actual motor involved in anterograde GlyR-gephyrin transport (Maas et al., 2009
) (Figure 3
B). It is therefore likely that synaptic transmission induces intracellular signalling that regulates the PTMs of transport tracks. These PTMs in turn determine in an activity-dependent manner, how much cargo may arrive at synaptic sites at a given time. Interestingly, live cell imaging revealed that strychnine-mediated GlyR blockade altered the percentage of mobile gephyrin, but not of GRIP1 particles over time, although both proteins act as cargo adaptors of the same motor protein (KIF5) and couple this motor to either GlyRs (Maas et al., 2009
) or AMPARs (Setou et al., 2002
), respectively. The currently available data therefore suggest that the actual cargo adapter, but not the motor itself, represents a critical factor that senses surface modifications at microtubule transport tracks, as induced through neuronal activity changes (Figure 3
).
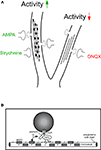
Figure 3. Activity-dependent polyglutamylation of tubulin alters intracellular transport. (A) Model of microtubule track changes through polyglutamylation (diagonal lines) and MAP2 binding (dark squares) upon altered neuronal activity. Increased activity, as induced through GlyR blockade (strychnine) or AMPAR activation (AMPA), interferes with gephyrin delivery into distal neurites (left). This effect is not observed upon neuronal activity reduction through AMPAR blockade (6,7-Dinitroquinoxaline-2,3-dione, DNQX) and can be prevented through functional depletion of neuronal polyglutamylase (the respective enzyme that adds polyglutamyl side chains to tubulin). Although it is unclear which modification is dominant, both represent negative signals for cargo delivery. (B) The individual cargo adaptor in the motor-cargo complex (gephyrin) is thought to mediate specificity of transport, as individual motor proteins transport multiple cargoes. Notably, KIF5-mediated transport of gephyrin is significantly reduced under strychnine conditions, whereas KIF5-mediated transport of GRIP1 (another cargo adaptor driven by the same motor) remains unaltered. The individual cargo adaptor within the transport complex (gephyrin) is therefore a candidate factor to sense modifications at the microtubule track surface (double arrow, question mark). Modified after Maas et al. (2009)
.
Regulatory mechanisms of this kind would be suitable to determine the intracellular transport direction of cargoes in a complex dendritic tree. If synapses in a local branch of a dendrite were to be highly active, cargo delivery into this region could be compromised due to microtubule PTMs that act as negative traffic signs or stop signals. In contrast, cargo transport into neurites, where reduced synaptic activity occurs, would be promoted. It will remain a future challenge to identify the intermediate components that mediate signalling between synaptic surface membranes and microtubules. Furthermore, it will have to be identified whether other posttranslational tubulin modifications, as for instance tubulin tyrosination (Konishi and Setou, 2009
), undergo activity-dependent regulation in neurons. In summary, intracellular transport critically participates in the steady-state process of synaptic molecule turnover in neurons and can be tuned by synaptic activity at different molecular levels, including cargo adaptor, motor protein and cytoskeletal track levels. However, it should be noted that the lateral diffusion of surface membrane receptors, also known to undergo activity-dependent regulation (Lévi et al., 2008
), might apply independent signalling pathways.
Membrane Insertion of GlyR
In theory, exocytosis of the GlyR-gephyrin complex could happen either at specific sites (such as the postsynaptic density), or at random locations of the plasma membrane, followed by subsequent incorporation in the synapse. There is a lack of data regarding this question, and only indirect evidence suggests that delivery of GlyR does not happen at synaptic sites (Rosenberg et al., 2001
). Regarding other receptors, GABAAR exocytosis occurs exclusively at extrasynaptic sites (Thomas et al., 2005
; Bogdanov et al., 2006
), and studies on the AMPAR GluR1 subunit showed that it is inserted in somatic and dendritic locations (Adesnik et al., 2005
; Yudowski et al., 2007
) and in the latter case, in the spine membrane (Park et al., 2004
). However, there can be variations among different receptors and among subunits of the same receptor, since the AMPAR GluR2 subunit has been shown to be inserted directly at synapses (Passafaro et al., 2001
).
GlyR Diffusion in the Plasma Membrane
Receptors are transmembrane proteins and, as for any other protein inserted in the plasma membrane, their movements undergo physical constraints. The fluid mosaic cell membrane model established more than thirty years ago by Singer and Nicolson (1972)
predicted “lateral and rotational freedom and random distribution of the components in the membrane.” Since then, it has been deeply remodelled and a new concept emerged where diffusion is far from being unrestricted (see Vereb et al., 2003
for a review). We now know that the plasma membrane is dynamic and structured, containing proteins that act as transient traps for other proteins (“pickets”: individual or multimolecular complexes, and lipid rafts microdomains) and obstacles that restrict their diffusion (“fences”, such as submembraneous filaments of cytoskeleton) (Dietrich et al., 2002
; Kusumi et al., 2005
). In addition, one should bear in mind that inhibitory postsynaptic membranes are highly viscous and crowded, more than excitatory ones (Renner et al., 2009
).
Once inserted in the plasma membrane, how does GlyR behave? In spite of what was known about the fluidity of the membrane, for a long time only immunocytochemistry of fixed tissue or cells could be used to visualize receptors and synapses. A static view of the synapse prevailed, revealing only the amount of receptors clustered in front of the presynaptic bouton at a given moment. This was also true for the putative receptors located in extrasynaptic regions, whose presence was suggested by electron microscopy observations and electrophysiological recordings. A more refined picture is now available, that takes into account both plasma membrane intrinsic features and time. Progress in videomicroscopy techniques and in particular the improvement of the CCD camera sensitivity, along with the use of fluorescent probes, made the study of the dynamics of living cells material possible. A study by Rosenberg et al. (2001)
provided the first evidence that surface membrane GlyRs, as located outside synaptic sites, had a dynamic behaviour. The authors followed the temporal sequence of GlyRα1 insertion on the plasma membrane and observed the initial insertion of GlyRs at the somatic membrane level. GlyR diffused from there to dendritic sites, at an estimated linear diffusion rate of 5 × 10−2 μm s−1. In a different approach, Meier et al. (2001)
used optical tweezers to direct a 0.5-μm-latex bead, coupled to antibodies against GlyR, and observed the trajectories of the bead moving on the surface of spinal cord neurons. This demonstrated for the first time that individual receptors were able to diffuse within the plasma membrane.
A real breakthrough on the study of membrane GlyR behaviour came later on from the use of antibodies coupled to quantum dots (QDs) (Dahan et al., 2003
). QDs are nanometer-sized probes that provide long-lasting fluorescence emission (Bawendi et al., 1990
; Bruchez et al., 1998
). Because of this property, they can be used to track identified molecules (single-particle tracking, SPT) for periods much longer than organic fluorescent dyes (20 min vs. 10 s). Trajectories of QDs recorded on living neurons revealed that, at the cell surface, GlyRs exchanged rapidly between extrasynaptic and synaptic compartments (Dahan et al., 2003
) (Figure 4
A,B). In extrasynaptic regions, QD-labelled GlyRs had characteristic Brownian, free-diffusing molecules trajectories. In the membrane context, these are passive random movements of proteins within the lipid bilayer that give a characteristic linear function of the mean square displacement (MSD) versus time (Figure 4
C). The mean diffusion coefficient can be inferred from the MSD curves, and revealed that GlyR explored an extrasynaptic area of 1 × 10−1 μm2 s−1. Within the synaptic compartment, two receptor populations could be distinguished: “rapid”-diffusing receptors (mean diffusion value of 7.3 × 10−2 μm2 s−1, about 20% of synaptic receptors) and “slow”-diffusing ones (1 × 10−3 μm2 s−1). The latter showed a biphasic MSD curve, typical of movements limited by other proteins inserted in or associated with the plasma membrane, and also called confined diffusion (Figure 4
C). Thus, the spontaneous trajectories of GlyRs showed that one receptor molecule can change from one diffusive state to another as it travels through distinct functional compartments, and that each behaviour has its own characteristics.
Figure 4. Diffusion properties of the glycine receptor. (A) Example of an individual GlyR-QDot trajectory exchanging between a synaptic (trace in green) and an extrasynaptic location (trace in blue). FM4-64-stained synapses are in red. (B) Time spent by the GlyR-QDot in the different compartments over a 40-s recording (same colour code). (C) Time-averaged MSD function of the QDot shown in (A). The two curves represent synaptic (green) and extrasynaptic (blue) portions of the trajectory. Curves are typical of confined (negatively bent) and free-diffusing molecules, respectively.
The same swapping behaviour between synaptic and extrasynaptic domains was observed for other receptors, namely the inhibitory GABAA receptor (Lévi et al., 2008
; Bannai et al., 2009
) and glutamatergic AMPA, NMDA and mGluR receptors (Sergé et al., 2002
; Tardin et al., 2003
; Groc et al., 2004
). For these receptors, diffusion rates in the different membrane domains were within similar ranges to those of GlyRs. A general pattern has thus emerged for both inhibitory and excitatory synapses. Receptors can be trapped by and released from an anchoring domain within seconds to minutes, leading to a rapid supply/depletion of receptor molecules at the synapse. This behaviour could account for the diffusion-trap model of receptor accumulation during synapse formation, driven by the progressive recruitment of scaffolding proteins from extrasynaptic to synaptic locations (Kirsch et al., 1993a
; Colin et al., 1998
; Rao et al., 1998
; Cottrell et al., 2000
; Dumoulin et al., 2000
; Borgdorff and Choquet, 2002
; Choquet and Triller, 2003
; Bellone and Nicoll, 2007
). Diffusion dynamics could also be responsible, along with changes in exocytosis and endocytosis rates, for the rapid regulation of receptor numbers required in synaptic plasticity events such as long-term potentiation (Shi et al., 1999
; Lu et al., 2001
; Earnshaw and Bressloff, 2006
; Lisman and Raghavachari, 2006
; Zhao et al., 2008
) and long-term depression (Carroll et al., 1999
; Luscher et al., 1999
; Earnshaw and Bressloff, 2006
) (see Newpher and Ehlers, 2009
, for a review). Finally, it has been demonstrated that the homeostatic regulation of the network activity itself was mediated at the cellular level through the lateral diffusion of receptors, as discussed in Section “Regulation of GlyR diffusion by neuronal activity” in this review.
Role of the Scaffolding Molecule Gephyrin in GlyR Diffusion
The previous results showed that the receptors have confined trajectories whenever located at synaptic sites. To assess whether there was a link between diffusion of GlyR and interaction with the scaffolding protein gephyrin, Meier et al. (2001)
transfected neurons with Venus-tagged Gephyrin (Ve-Ge) and a modified GlyRα1 construct able to bind gephyrin (GlyRa1bgb; Meier et al., 2000
). Trajectories were followed thanks to a latex bead coupled to antibodies against GlyR. This approach showed that GlyRα1βgb clusters exhibited lateral diffusion along the plasma membrane and, in the absence of gephyrin, trajectories were typical of free diffusing molecules (diffusion coefficient 2.5 × 10−2 μm2 s−1). In neurons co-transfected with Ve-Ge, particles alternated between fast (1.1 × 10−2 μm2 s−1) and slow (1.1 × 10−3 μm2 s−1) diffusion rates, depending on the absence or presence of gephyrin clusters, respectively. Interaction between the receptor and the submembraneous protein accounted for the confined movements recorded. When compared with endogenous receptor diffusion results from Q-Dot trajectories (Dahan et al., 2003
), values for slow-diffusing receptors were similar in the two experiments. However, extrasynaptic endogenous receptors diffused ten times faster than GlyRα1βg non associated with gephyrin clusters (Meier et al., 2001
). This discrepancy could be due to the difference in methodology (Q Dot vs. 500 nm latex bead). Alternatively, it could be explained by a difference in the membrane composition of spinal cord neurons, since Dahan et al. used mature neurons, while Meier et al. used immature ones (2–3 DIV). Indeed, changes in lipids have been documented throughout maturation of neurons in culture (Prinetti et al., 2001
), and cholesterol depletion was demonstrated to change the diffusion rates of GABAAR in hippocampal neurons in culture (Renner et al., 2009
).
A complementary study further investigated the role of gephyrin in GlyR diffusion, in particular outside synaptic locations (Ehrensperger et al., 2007
). As in the previous paradigm, neurons were co-transfected with Ve-Ge and GlyRα1βgb constructions, and this time trajectories of GlyRα1βgb were tracked by use of QDots. The diffusion coefficient found for GlyRα1βgb associated with gephyrin confirmed the slow diffusing rates from Meier et al. (2001)
. Two new findings arised from this study. First, the diffusion of a native form of GlyRa1 (not binding gephyrin) was 20 times faster than that of GlyRα1βgb in cells cotransfected with Ve-Ge, indicating that, even outside visible gephyrin clusters, gephyrin restricts GlyR dynamics. This is coherent with the fact that GlyR and gephyrin associate early after synthesis and during trafficking (Hanus et al., 2004
; Maas et al., 2006
), and with the presence of their complex at the plasma membrane outside synaptic locations. Second, receptors associated with Ve-Ge could either be stable (high confinement, slow diffusion), or swap between different Ve-Ge clusters during the 40-s recordings. This observations lead to the concept that receptor stabilization by clusters of gephyrin is only transient. However, as receptors can escape from a given gephyrin domain, the diffusive behaviour suggested the existence of multiple association states between the two (Ehrensperger et al., 2007)
. Transient stabilization by scaffolding proteins also appear to be the rule for other receptors. Jacob et al. (2005)
demonstrated that GABAAR diffusion properties also relied on the presence of gephyrin clusters, and reversible interactions in a short time-scale between receptors and scaffolds have also been shown for AMPA receptors with PSD-95 and stargazin (Bats et al., 2007
) and for mGluR5 with Homer (Sergé et al., 2002
).
Do Synaptic Scaffolds also Show Dynamic Behaviour?
Glycine receptor exchanges between synaptic and extrasynaptic compartments, and interaction with the scaffolding protein gephyrin stabilizes receptor movements. But how stable is the scaffold itself? To address this issue, Hanus et al. (2006)
recorded the movements of gephyrin in spinal cord neurons transfected with Ve-Ge. Synaptic clusters of Ve-Ge displayed submicrometric lateral motion around a central position, with a diffusion rate of 7.1 × 10−4 μm2 s−1. This value is within the same range of that of the “slow” endogenous synaptic receptors, but very different from the “fast” synaptic ones (7.3 × 10−2μm2 s−1, Dahan et al., 2003
). Thus, movements of receptors and movements of gephyrin should be considered as distinct but simultaneous phenomena. Fluorescence recovery after photobleaching (FRAP) experiments proved valuable to further investigate the behaviour of populations of gephyrin molecules within scaffolds. Clusters of Ve-Ge or mRFP-gephyrin were bleached (Calamai et al., 2009
) and in the two cases, 40 % of the bleached molecules were replaced by non bleached ones within 30 min (reviewed in Specht and Triller, 2008
). Taken together, these results show that gephyrin clusters move and that, while doing so, molecules of gephyrin exchange between different pools. Molecules being added and removed in a regular fashion to/from the structure formed by gephyrin underneath the synapse could explain that receptors swapping from one domain to another would still be attached to gephyrin molecules even outside synaptic locations, as demonstrated by Ehrensperger et al. (2007)
. In excitatory synapses, scaffolding proteins also exchange in a dynamic fashion. In particular, CamKII, Homer, GKAP and Shank have an important mobile pool, while PSD95 is relatively stable at the PSD, as shown by FRAP experiments (Gray et al., 2006
; Kuriu et al., 2006
; Sharma et al., 2006
).
However, the question whether gephyrin dynamics could influence the dynamics of GlyR remained to be addressed. The studies cited previously were undertaken with the full-length isoform of gephyrin (Ge, corresponding to the p1 clone in other publications). Still, other splice variants of gephyrin exist in the CNS that can or cannot bind GlyR, and have oligomerization properties different than those described in Figure 1
(Bedet et al., 2006
; Saiyed et al., 2007
). Calamai et al. (2009)
investigated how changes in gephyrin dynamics, through the oligomerization of different variants and deletion mutants, influenced GlyR clustering and diffusion. At extrasynaptic regions, analysis of SPT trajectories of endogenous GlyR showed that the diffusion rates in neurons transfected with the variants that lacked optimal polymerization properties were significantly higher than in neurons transfected with full-length Ve-Ge (1–2 × 10−2 μm2 s−1 vs. 6.3 × 10−3 μm2 s−1, respectively). Thus, gephyrin–gephyrin association dynamics do influence the lateral difffusion of GlyR outside the synapse. Such a modulation could not be assessed at synaptic locations since the variants seem to be excluded from mixed synaptic clusters in neurons co-transfected with Ge and the variants (Calamai et al., 2009
). However, a direct implication of the integrity of the multimolecular stargazin-PSD95-AMPAR complex on the residency time of AMPA receptors at the synapse has been demonstrated by SPT, in a model of a mutant mouse, deficient for the stargazin-PSD95 interaction (Bats et al., 2007
; see Newpher and Ehlers, 2008
).
Role of Cytoskeletal Elements in GlyR and Gephyrin Dynamics
Native GlyRs bind to gephyrin through a direct interaction between the GlyRβ subunit M3-M4 loop and the E domain of gephyrin (Kirsch and Betz, 1995
; Kneussel et al., 1999
; Kim et al., 2006
), and gephyrin in turn associates with microtubules. Gephyrin also interacts indirectly with the actin microfilament cytoskeleton through proteins like profilin and Mena/Vasp (Mammoto et al., 1998
; Giesemann et al., 2003
), and with GTPases through collybistin (Xiang et al., 2006
). Actin and microtubules appear thus as good candidates to modulate gephyrin and/or GlyR diffusion, as they do for gephyrin trafficking (Maas et al., 2009
). A number of studies indicate that these two components are indeed involved in the regulation of synaptic components density. In particular, a reduction in size and immunoreactivity intensity of gephyrin and GlyR clusters was observed after microtubule depolymerization, correlated with a decrease in the amplitude of glycinergic mISPCs (Kirsch and Betz, 1995
; van Zundert et al., 2004
; Charrier et al., 2006
). A similar response was observed by immunocytochemistry after actin network disruption (Charrier et al., 2006
).
The effects of actin filament and microtubule depolymerization on the lateral diffusion of GlyRs (Charrier et al., 2006
) and gephyrin (Hanus et al., 2006
) were studied by use of the drugs latrunculin and nocodazole, respectively. SPT analysis revealed that, after addition of latrunculin or nocodazole, GlyR diffusion was significantly increased. The receptor explored larger areas of extrasynaptic membrane and exchanged more frequently between extrasynaptic and synaptic compartments. Within synapses, an increase in GlyR diffusion coefficients was seen after latrunculin treatment only (1 × 10−2 μm2 s−1 vs. 1 × 10−3 μm2 s−1 for control). Regarding gephyrin, the synaptic clusters diffused less after microfilament depolymerization, but showed increased MSD values after microtubules disruption. Taken together, these data suggest that, at synaptic locations, actin contributes simultaneously to the confinement of the receptor and to the mobility of gephyrin, regulating the organization of confinement sites (also see Renner et al., 2009
). The interactions between gephyrin, GlyR and the cytoskeleton that regulate diffusion appear to be complex and not fully understood. A simpler view emerges from excitatory synapses, probably because mostly actin is present in spines -even though recent data suggest a role for microtubules in spine morphology plasticity (Jaworski et al., 2009
). Allison et al. (2000)
showed that actin depolymerization reduced the number of AMPAR clusters at both synaptic and extrasynaptic locations, while a reduction was only observed for synaptic NMDAR aggregates. Receptor diffusion was not assessed by SPT in these conditions, but the mobile fraction of scaffolding proteins GKAP, Shank and Homer, that exchanged in a dynamic fashion as seen by FRAP experiments, disappeared after lantrunculin administration (Kuriu et al., 2006
). No effect was observed in PSD 95 distribution, which confirms the results obtained by Usui et al. (2003)
.
Regulation of GlyR Diffusion by Neuronal Activity
Since receptor diffusion mechanisms appear to be controlled by a range of interacting factors, an important issue is whether activity of the network itself can regulate its behaviour. This question was assessed for GlyR dynamics by SPT in spinal cord neurons, where modifications were induced by administration of tetrodotoxin, alone or in combination with GlyR, GABAAR, AMPAR and NMDAR antagonists (Lévi et al., 2008
). Synaptic transmission was shown to control GlyR lateral diffusion via activation of the NMDAR, leading to a greater confinement of synaptic and extrasynaptic receptors and slower diffusion rates. This was correlated with increased levels of GlyR in synaptic clusters and increased amplitude of glycinergic mIPSCs. Thus, global excitatory activity directly controls efficiency of transmission through receptor lateral diffusion and clustering, and suggests an implication of GlyR diffusion in homeostatic regulation (i.e. the mechanisms through which a neuron adapts its inhibition when the excitation level is modified). In this case, changes in diffusion rates could be a very early step in network homeostasis. A study by Bannai et al. (2009)
on GABAAR in hippocampal neurons revealed that upon pharmacological increase of excitatory activity, the synaptic and extrasynaptic diffusion coefficients of GABAAR were increased, and that they were correlated with reduced confinement areas and decreased amplitude of the recorded mIPSCs. These major findings demonstrate that network excitatory activity regulates GlyR and GABAR diffusions in opposite directions, highlighting a functional regulatory difference between the two inhibitory receptors. Interestingly, in mixed inhibitory synapses (containing both GlyR and GABAAR) of spinal cord neurons (Lévi et al., 1999
; Dumoulin et al., 2000
), no effect of excitatory activity mediated by NMDAR was observed on the lateral diffusion of GABAAR (Lévi et al., 2008
). Finally, among the glutamatergic receptors, AMPAR but not NMDAR diffusion dynamics were found to be activity-dependent (Tardin et al., 2003
; Groc et al., 2004
, 2006
; Ehlers et al., 2007
). In conclusion, homeostatic adaptation by receptor diffusion regulation represents a mechanism used by several neurotransmitter systems to drive quick changes in the distribution of receptor molecules between extrasynaptic and synaptic compartments and tune individual synaptic strength to the network activity. Noteworthy, receptor diffusion regulation by global activity has been shown to rely on calcium signalling (Borgdorff and Choquet, 2002
; Tardin et al., 2003
; Lévi et al., 2008
; Bannai et al., 2009
).
Analysis of receptor trapping and release events from postsynaptic gephyrin-containing scaffolds led to the view of receptor dynamics as an equilibrium state where scaffolds behave simultaneously as acceptors and donors of receptors. This interaction can be chemically characterized by association (kon) and dissociation constants (koff) (Figure 5
A). Multiple association states exist between the two proteins, which can be summarized in the equilibrium representation in Figure 5
B (Choquet and Triller, 2003
; Holcman and Triller, 2006
; Ehrensperger et al., 2007
; Triller and Choquet, 2008
). Receptors can associate/dissociate with the scaffold molecule within the synapse or outside of it, and diffuse together on the cell membrane. However, from the results presented here, we can expect the receptor forms non associated with gephyrin (either at extrasynaptic or at synaptic sites, and defined as Ro and Ri in Figure 5
, respectively) to represent only a small proportion of the receptor pool. Once present in an associated form at synaptic sites (RiSi), a higher degree of stabilization of the complex could be reached (equilibrium 5, asterisk). Such a dynamic equilibrium starts unravelling the apparent paradox in which the function of a synapse requires it to be stable in time (metastability), and still its receptors units show instability by quickly getting in and out of it. This model, obtained through results of GlyR diffusion, is also supported by and can be generalized for other receptors.
Figure 5. Model of receptor diffusion and stabilization at synapses. (A) Receptor exchanges between extrasynaptic and synaptic domains. The rates of entry and exit from gephyrin clusters define the kon and koff, respectively. (B) Representation of the different paths leading to the stabilization of GlyR by gephyrin clusters. Association of receptor (R) and its scaffolding protein gephyrin (S) can occur outside (equilibrium 1) or inside (equilibrium 4) synaptic sites. Once within clusters, receptor-scaffold complexes may reach a higher level of stabilization (equilibrium 5, dark gray). The index “i” indicates the inside and the index “o” the outside of the synaptic domain (light gray area) (modified from Ehrensperger et al., 2007
).
Based on the previous model, Sekimoto and Triller (2009)
developed a new general mesoscopic model, considering the highly compartmentalized structure of the synapse. The postsynaptic domain was spatially defined as a three-layer (membrane, sub-membrane, cytoplasm), two-zone (synaptic, extrasynaptic) model where all interactions between receptors and scaffolding proteins occurred. Within this model, the authors considered both the concentrations and chemical potentials of receptor and scaffolding protein. This resulted in a highly cooperative thermodynamic model of postsynaptic domain stability. Changing the concentrations of receptor and scaffolding molecules in a given compartment, or modifying the interaction between them, led to discrete modifications of receptor numbers at synapses. Another important issue arising from this work is that stabilization is a reciprocal mechanism: receptors are stabilized by their interaction with the scaffold, but the opposite is also true. This notion can be particular relevant during synapse formation and plasticity, since no player on its own could be responsible for synapse construction and adaptive modifications.
In conclusion, we have focused on the review and discussion of data regarding the trafficking of the GlyR inside the neuron and at the plasma membrane. GlyRs associate intracellularly after synthesis with gephyrin, and the complex travels to the membrane applying the microtubule-dependent motor protein KIF5. Transport can be regulated by neuronal activity through phosphorylation of motor proteins or through PTMs of tubulin. Once inserted in the membrane, the GlyR-gephyrin complex is able to diffuse to synaptic sites where it is stabilized. However, stabilization is transient, since molecules can exchange rapidly between different compartments, and relies on the integrity of the cytoskeleton. Theoretical models indicate that the transient stabilization of the receptor by gephyrin and the turnover of the latter is however compatible with a “dynamic stabilization” of the postsynaptic domain. Finally, network activity influences both intracellular transport and the diffusion dynamics of GlyRs, which adapts its numbers at synapses to match activity changes in a homeostatic fashion.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are indebt to Sabine Lévi, Patricia Machado and Christian Vannier for providing figure material. Work in the lab of M. Kneussel was supported by the University of Hamburg, DFG grant FG885-KN556/4-1 and an award of the Chica and Heinz Schaller Foundation. Work in the lab of A. Triller was supported by Inserm and the Ecole Normale Supérieure, Paris.
Giesemann, T., Schwarz, G., Nawrotzki, R., Berhorster, K., Rothkegel, M., Schluter, K., Schrader, N., Schindelin, H., Mendel, R. R., Kirsch, J., and Jockusch, B. M. (2003). Complex formation between the postsynaptic scaffolding protein gephyrin, profilin, and Mena: a possible link to the microfilament system. J. Neurosci. 23, 8330–8339.
Ikegami, K., Heier, R. L., Taruishi, M., Takagi, H., Mukai, M., Shimma, S., Taira, S., Hatanaka, K., Morone, N., Yao, I., Campbell, P. K., Yuasa, S., Janke, C., MacGregor, G. R., and Setou, M. (2007). Loss of alphatubulin polyglutamylation in ROSA22 mice is associated with abnormal targeting of KIF1A and modulated synaptic function. Proc. Natl. Acad. Sci. U.S.A. 104, 3213–3218.
Jaworski, J., Kapitein, L. C., Gouveia, S. M., Dortland, B. R., Wulf, P. S., Grigoriev, I., Camera, P., Spangler, S. A., Di Stefano, P., Demmers, J., Krugers, H., Defilippi, P., Akhmanova, A., and Hoogenraad, C. C. (2009). Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61, 85–100.
Kusumi, A., Nakada, C., Ritchie, K., Murase, K., Suzuki, K., Murakoshi, H., Kasai, R. S., Kondo, J., and Fujiwara, T. (2005). Paradigm shift of the plasma membrane concept from the two dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct. 34, 351–378.
Traer, C. J., Rutherford, A. C., Palmer, K. J., Wassmer, T., Oakley, J., Attar, N., Carlton, J. G., Kremerskothen, J., Stephens, D. J., and Cullen, P. J. (2007). SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat. Cell Biol. 9, 1370–1380.