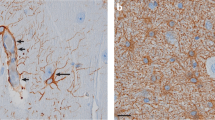
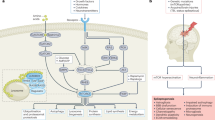
Abstract
Epilepsy is the third most common chronic brain disorder, and is characterized by an enduring predisposition to generate seizures. Despite progress in pharmacological and surgical treatments of epilepsy, relatively little is known about the processes leading to the generation of individual seizures, and about the mechanisms whereby a healthy brain is rendered epileptic. These gaps in our knowledge hamper the development of better preventive treatments and cures for the ≈30% of epilepsy cases that prove resistant to current therapies. Here, we focus on the rapidly growing body of evidence that supports the involvement of inflammatory mediators—released by brain cells and peripheral immune cells—in both the origin of individual seizures and the epileptogenic process. We first describe aspects of brain inflammation and immunity, before exploring the evidence from clinical and experimental studies for a relationship between inflammation and epilepsy. Subsequently, we discuss how seizures cause inflammation, and whether such inflammation, in turn, influences the occurrence and severity of seizures, and seizure-related neuronal death. Further insight into the complex role of inflammation in the generation and exacerbation of epilepsy should yield new molecular targets for the design of antiepileptic drugs, which might not only inhibit the symptoms of this disorder, but also prevent or abrogate disease pathogenesis.
Key Points
-
Epilepsies of various etiologies not classically linked to immunological dysfunction can be associated with inflammation resulting from increased levels of inflammatory mediators in the brain
-
Inflammatory mediators can be produced by glia, neurons, endothelial cells of the blood–brain barrier, and peripheral immune cells
-
Brain inflammation might contribute to the onset and perpetuation of seizures in a variety of epilepsies
-
Experimental and clinical research is required to generate novel therapeutic anti-inflammatory approaches that ameliorate seizures and modify the underlying pathophysiology of epilepsy
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Duncan, J. S., Sander, J. W., Sisodiya, S. M. & Walker, M. C. Adult epilepsy. Lancet 367, 1087–1100 (2006).
Perucca, E., French, J. & Bialer, M. Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol. 6, 793–804 (2007).
Rogawski, M. A. & Loscher, W. The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 5, 553–564 (2004).
Pitkanen, A. & Sutula, T. P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 1, 173–181 (2002).
Vezzani, A. & Granata, T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia 46, 1724–1743 (2005).
Vezzani, A. & Baram, T. Z. New roles for interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Curr. 7, 45–50 (2007).
Riazi, K., Galic, M. A. & Pittman, Q. J. Contributions of peripheral inflammation to seizure susceptibility: cytokines and brain excitability. Epilepsy Res. 89, 34–42 (2010).
Choi, J. et al. Cellular injury and neuroinflammation in children with chronic intractable epilepsy. J. Neuroinflammation 6, 38 (2009).
Riikonen, R. Infantile spasms: therapy and outcome. J. Child Neurol. 19, 401–404 (2004).
Wheless, J. W., Clarke, D. F., Arzimanoglou, A. & Carpenter, D. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord. 9, 353–412 (2007).
Wirrell, E., Farrell, K. & Whiting, S. The epileptic encephalopathies of infancy and childhood. Can. J. Neurol. Sci. 32, 409–418 (2005).
Dubé, C. M., Brewster, A. L., Richichi, C., Zha, Q. & Baram, T. Z. Fever, febrile seizures and epilepsy. Trends Neurosci. 30, 490–496 (2007).
Dinarello, C. A. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J. Endotoxin Res. 10, 201–222 (2004).
Rasmussen, T., Olsewski, J. & Lloyd-Smith, D. Focal seizures due to chronic localized encephalitis. Neurology 8, 435–445 (1958).
Bien, C. G. et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology 69, 1236–1244 (2007).
Dalmau, J. et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 7, 1091–1098 (2008).
Vincent, A. & Bien, C. G. Anti-NMDA-receptor encephalitis: a cause of psychiatric, seizure, and movement disorders in young adults. Lancet Neurol. 7, 1074–1075 (2008).
Aarli, J. A. Epilepsy and the immune system. Arch. Neurol. 57, 1689–1692 (2000).
Ravizza, T., Balosso, S., Aronica, E. & Vezzani, A. in Epilepsy: Mechanisms, Models, and Translational Perpsectives Ch. 4 (eds Rho, J. M. et al.) 45–59 (CRC Press, Boca Raton, 2010).
Campbell, I. L. et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl Acad. Sci. USA 90, 10061–10065 (1993).
Vezzani, A. et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc. Natl Acad. Sci. USA 97, 11534–11539 (2000).
Balosso, S. et al. Tumor necrosis factor-α inhibits seizures in mice via p75 receptors. Ann. Neurol. 57, 804–812 (2005).
Ravizza, T. et al. Inactivation of caspase-1 in rodent brain: a novel anticonvulsive strategy. Epilepsia 47, 1160–1168 (2006).
Samland, H. et al. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J. Neurosci. Res. 73, 176–187 (2003).
Akassoglou, K., Probert, L., Kontogeorgos, G. & Kollias, G. Astrocyte-specific but not neuron-specific transmembrane TNF triggers inflammation and degeneration in the central nervous system of transgenic mice. J. Immunol. 158, 438–445 (1997).
Kelley, K. A. et al. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am. J. Pathol. 155, 995–1004 (1999).
De Sarro, G. et al. Seizure susceptibility to various convulsant stimuli of knockout interleukin-6 mice. Pharmacol. Biochem. Behav. 77, 761–766 (2004).
Ransohoff, R. M., Kivisakk, P. & Kidd, G. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 3, 569–581 (2003).
Banks, W. A. & Erickson, M. A. The blood–brain barrier and immune function and dysfunction. Neurobiol. Dis. 37, 26–32 (2010).
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C. & Gage, F. H. Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 (2010).
Nguyen, M. D., Julien, J. P. & Rivest, S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 3, 216–227 (2002).
Appel, S. H., Beers, D. R. & Henkel, J. S. T cell–microglial dialog in Parkinson's disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol. 31, 7–17 (2009).
Allan, S. M., Tyrrell, P. J. & Rothwell, N. J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 5, 629–640 (2005).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 (2001).
Bartfai, T. et al. Interleukin-1 system in CNS stress: seizures, fever, and neurotrauma. Ann. N. Y. Acad. Sci. 1113, 173–177 (2007).
Allan, S. M. & Rothwell, N. J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2, 734–744 (2001).
Bartfai, T. & Schultzberg, M. Cytokines in neuronal cell types. Neurochem. Int. 22, 435–444 (1993).
Wilson, E. H., Weninger, W. & Hunter, C. A. Trafficking of immune cells in the central nervous system. J. Clin. Invest. 120, 1368–1379 (2010).
Szekanecz, Z. & Koch, A. E. Chemokines and angiogenesis. Curr. Opin. Rheumatol. 13, 202–208 (2001).
Semple, B. D., Kossmann, T. & Morganti-Kossmann, M. C. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 30, 459–473 (2010).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Mantovani, A., Locati, M., Vecchi, A., Sozzani, S. & Allavena, P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 22, 328–336 (2001).
Baker, B. J., Akhtar, L. N. & Benveniste, E. N. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 30, 392–400 (2009).
Khuu, C. H., Barrozo, R. M., Hai, T. & Weinstein, S. L. Activating transcription factor 3 (ATF3) represses the expression of CCL4 in murine macrophages. Mol. Immunol. 44, 1598–1605 (2007).
Ho, H. H., Antoniv, T. T., Ji, J. D. & Ivashkiv, L. B. Lipopolysaccharide-induced expression of matrix metalloproteinases in human monocytes is suppressed by IFN-γ via superinduction of ATF-3 and suppression of AP-1. J. Immunol. 181, 5089–5097 (2008).
Blobe, G. C., Schiemann, W. P. & Lodish, H. F. Role of transforming growth factor β in human disease. N. Engl. J. Med. 342, 1350–1358 (2000).
Sapolsky, R., Rivier, C., Yamamoto, G., Plotsky, P. & Vale, W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238, 522–524 (1987).
Rothwell, N. J. CRF is involved in the pyrogenic and thermogenic effects of interleukin 1 beta in the rat. Am. J. Physiol. 256, E111–E115 (1989).
Elenkov, I. J., Webster, E. L., Torpy, D. J. & Chrousos, G. P. Stress, corticotropin-releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann. N. Y. Acad. Sci. 876, 1–13 (1999).
Tracey, K. J. The inflammatory reflex. Nature 420, 853–859 (2002).
Najjar, S., Bernbaum, M., Lai, G. & Devinsky, O. Immunology and epilepsy. Rev. Neurol. Dis. 5, 109–116 (2008).
Rogers, S. W. et al. Autoantibodies to glutamate receptor GluR3 in Rasmussen's encephalitis. Science 265, 648–651 (1994).
Mantegazza, R. et al. Antibodies against GluR3 peptides are not specific for Rasmussen's encephalitis but are also present in epilepsy patients with severe, early onset disease and intractable seizures. J. Neuroimmunol. 131, 179–185 (2002).
Watson, R. et al. Absence of antibodies to glutamate receptor type 3 (GluR3) in Rasmussen encephalitis. Neurology 63, 43–50 (2004).
Bien, C. G. et al. Destruction of neurons by cytotoxic T cells: a new pathogenic mechanism in Rasmussen's encephalitis. Ann. Neurol. 51, 311–318 (2002).
Baranzini, S. E., Laxer, K., Bollen, A. & Oksenberg, J. R. Gene expression analysis reveals altered brain transcription of glutamate receptors and inflammatory genes in a patient with chronic focal (Rasmussen's) encephalitis. J. Neuroimmunol. 128, 9–15 (2002).
Pardo, C. A. et al. The pathology of Rasmussen syndrome: stages of cortical involvement and neuropathological studies in 45 hemispherectomies. Epilepsia 45, 516–526 (2004).
Wirenfeldt, M. et al. Increased activation of Iba1+ microglia in pediatric epilepsy patients with Rasmussen's encephalitis compared with cortical dysplasia and tuberous sclerosis complex. Neurobiol. Dis. 34, 432–440 (2009).
Takahashi, Y., Mine, J., Kubota, Y., Yamazaki, E. & Fujiwara, T. A substantial number of Rasmussen syndrome patients have increased IgG, CD4+ T cells, TNFα, and granzyme B in CSF. Epilepsia 50, 1419–1431 (2009).
Vincent, A., Irani, S. R. & Lang, B. The growing recognition of immunotherapy-responsive seizure disorders with autoantibodies to specific neuronal proteins. Curr. Opin. Neurol. 23, 144–150 (2010).
Crespel, A. et al. Inflammatory reactions in human medial temporal lobe epilepsy with hippocampal sclerosis. Brain Res. 952, 159–169 (2002).
Aronica, E. et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol. Dis. 26, 497–511 (2007).
Ravizza, T. et al. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 29, 142–160 (2008).
van Gassen, K. L. et al. Possible role of the innate immunity in temporal lobe epilepsy. Epilepsia 49, 1055–1065 (2008).
Maldonado, M. et al. Expression of ICAM-1, TNF-α, NFκB, and MAP kinase in tubers of the tuberous sclerosis complex. Neurobiol. Dis. 14, 279–290 (2003).
Boer, K. et al. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 78, 7–21 (2008).
Boer, K. et al. Gene expression analysis of tuberous sclerosis complex cortical tubers reveals increased expression of adhesion and inflammatory factors. Brain Pathol. 20, 704–719 (2010).
Iyer, A. M. et al. Tissue plasminogen activator and urokinase plasminogen activator in human epileptogenic pathologies. Neuroscience 19, 929–945 (2010).
Iyer, A. et al. Evaluation of the innate and adaptive immunity in type I and type II focal cortical dysplasias. Epilepsia 51, 1763–1773 (2010).
Ravizza, T. et al. The IL-1β system in epilepsy-associated malformations of cortical development. Neurobiol. Dis. 24, 128–143 (2006).
Minami, M., Kuraishi, Y. & Satoh, M. Effects of kainic acid on messenger RNA levels of IL-1β, IL-6, TNF-α and LIF in the rat brain. Biochem. Biophys. Res. Commun. 176, 593–598 (1991).
Vezzani, A. et al. Interleukin-1β immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J. Neurosci. 19, 5054–5065 (1999).
De Simoni, M. G. et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur. J. Neurosci. 12, 2623–2633 (2000).
Turrin, N. P. & Rivest, S. Innate immune reaction in response to seizures: implications for the neuropathology associated with epilepsy. Neurobiol. Dis. 16, 321–334 (2004).
Eriksson, C., Tehranian, R., Iverfeldt, K., Winblad, B. & Schultzberg, M. Increased expression of mRNA encoding interleukin-1β and caspase-1, and the secreted isoform of interleukin-1 receptor antagonist in the rat brain following systemic kainic acid administration. J. Neurosci. Res. 60, 266–279 (2000).
Voutsinos-Porche, B. et al. Temporal patterns of the cerebral inflammatory response in the rat lithium-pilocarpine model of temporal lobe epilepsy. Neurobiol. Dis. 17, 385–402 (2004).
Jung, K. H. et al. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol. Dis. 23, 237–246 (2006).
Lee, B., Dziema, H., Lee, K. H., Choi, Y. S. & Obrietan, K. CRE-mediated transcription and COX-2 expression in the pilocarpine model of status epilepticus. Neurobiol. Dis. 25, 80–91 (2007).
Gorter, J. A. et al. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J. Neurosci. 26, 11083–11110 (2006).
Polascheck, N., Bankstahl, M. & Loscher, W. The COX-2 inhibitor parecoxib is neuroprotective but not antiepileptogenic in the pilocarpine model of temporal lobe epilepsy. Exp. Neurol. 224, 219–233 (2010).
Holtman, L. et al. Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res. 84, 56–66 (2009).
Dhote, F. et al. Prolonged inflammatory gene response following soman-induced seizures in mice. Toxicology 238, 166–176 (2007).
Yoshikawa, K., Kita, Y., Kishimoto, K. & Shimizu, T. Profiling of eicosanoid production in the rat hippocampus during kainic acid-induced seizure: dual phase regulation and differential involvement of COX-1 and COX-2. J. Biol. Chem. 281, 14663–14669 (2006).
Rozovsky, I. et al. Selective expression of clusterin (SGP-2) and complement C1qB and C4 during responses to neurotoxins in vivo and in vitro. Neuroscience 62, 741–758 (1994).
Kulkarni, S. K. & Dhir, A. Cyclooxygenase in epilepsy: from perception to application. Drugs Today (Barc.) 45, 135–154 (2009).
Vezzani, A., Balosso, S. & Ravizza, T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav. Immun. 22, 797–803 (2008).
Fabene, P. F., Bramanti, P. & Constantin, G. The emerging role for chemokines in epilepsy. J. Neuroimmunol. 224, 22–27 (2010).
Xu, J. H. et al. CCR3, CCR2A and macrophage inflammatory protein (MIP)-1a, monocyte chemotactic protein-1 (MCP-1) in the mouse hippocampus during and after pilocarpine-induced status epilepticus (PISE). Neuropathol. Appl. Neurobiol. 35, 496–514 (2009).
Foresti, M. L. et al. Chemokine CCL2 and its receptor CCR2 are increased in the hippocampus following pilocarpine-induced status epilepticus. J. Neuroinflammation 6, 40 (2009).
Manley, N. C., Bertrand, A. A., Kinney, K. S., Hing, T. C. & Sapolsky, R. M. Characterization of monocyte chemoattractant protein-1 expression following a kainate model of status epilepticus. Brain Res. 1182, 138–143 (2007).
Wu, Y. et al. Expression of monocyte chemoattractant protein-1 in brain tissue of patients with intractable epilepsy. Clin. Neuropathol. 27, 55–63 (2008).
Fabene, P. F. et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 14, 1377–1383 (2008).
Librizzi, L. et al. Expression of adhesion factors induced by epileptiform activity in the endothelium of the isolated guinea pig brain in vitro. Epilepsia 48, 743–751 (2007).
Librizzi, L., Ravizza, T., Vezzani, A. & de Curtis, M. Expression of IL-1β induced by epileptiform activity in the isolated guinea pig brain in vitro. Presented at the 9th European Congress on Epileptology (Rhodes, 2010).
Dubé, C. M. et al. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J. Neurosci. 30, 7484–7494 (2010).
van Vliet, E. A. et al. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 130, 521–534 (2007).
Marchi, N. et al. Seizure-promoting effect of blood–brain barrier disruption. Epilepsia 48, 732–742 (2007).
Oby, E. & Janigro, D. The blood–brain barrier and epilepsy. Epilepsia 47, 1761–1774 (2006).
Marcon, J. et al. Age-dependent vascular changes induced by status epilepticus in rat forebrain: implications for epileptogenesis Neurobiol. Dis. 34, 121–132 (2009).
Majores, M., Eils, J., Wiestler, O. D. & Becker, A. J. Molecular profiling of temporal lobe epilepsy: comparison of data from human tissue samples and animal models. Epilepsy Res. 60, 173–178 (2004).
Lukasiuk, K., Dabrowski, M., Adach, A. & Pitkanen, A. Epileptogenesis-related genes revisited. Prog. Brain Res. 158, 223–241 (2006).
Xiong, Z. Q., Qian, W., Suzuki, K. & McNamara, J. O. Formation of complement membrane attack complex in mammalian cerebral cortex evokes seizures and neurodegeneration. J. Neurosci. 23, 955–960 (2003).
Berg, A. T., Darefsky, A. S., Holford, T. R. & Shinnar, S. Seizures with fever after unprovoked seizures: an analysis in children followed from the time of a first febrile seizure. Epilepsia 39, 77–80 (1998).
Zetterstrom, M., Sundgren-Andersson, A. K., Ostlund, P. & Bartfai, T. Delineation of the proinflammatory cytokine cascade in fever induction. Ann. N. Y. Acad. Sci. 856, 48–52 (1998).
Gatti, S., Vezzani, A. & Bartfai, T. in Febrile Seizures Ch. 12 (eds Baram, T. Z. & Shinnar, S.) 169–184 (Academic Press, San Diego, 2002).
Cartmell, T., Luheshi, G. N. & Rothwell, N. J. Brain sites of action of endogenous interleukin-1 in the febrile response to localized inflammation in the rat. J. Physiol. 518, 585–594 (1999).
Dubé, C., Vezzani, A., Behrens, M., Bartfai, T. & Baram, T. Z. Interleukin-1β contributes to the generation of experimental febrile seizures. Ann. Neurol. 57, 152–155 (2005).
Virta, M., Hurme, M. & Helminen, M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia 43, 920–923 (2002).
Haspolat, S. et al. Interleukin-1β, tumor necrosis factor-α, and nitrite levels in febrile seizures. J. Child Neurol. 17, 749–751 (2002).
Ichiyama, T., Nishikawa, M., Yoshitomi, T., Hayashi, T. & Furukawa, S. Tumor necrosis factor-α, interleukin-1β, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures. Comparison with acute encephalitis/encephalopathy. Neurology 50, 407–411 (1998).
Lahat, E., Livne, M., Barr, J. & Katz, Y. Interleukin-1β levels in serum and cerebrospinal fluid of children with febrile seizures. Pediatr. Neurol. 17, 34–36 (1997).
Heida, J. G. & Pittman, Q. J. Causal links between brain cytokines and experimental febrile convulsions in the rat. Epilepsia 46, 1906–1913 (2005).
French, J. A. et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann. Neurol. 34, 774–780 (1993).
Scantlebury, M. H. & Heida, J. G. Febrile seizures and temporal lobe epileptogenesis. Epilepsy Res. 89, 27–33 (2010).
Dubé, C. et al. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain 129, 911–922 (2006).
Sayyah, M., Javad-Pour, M. & Ghazi-Khansari, M. The bacterial endotoxin lipopolysaccharide enhances seizure susceptibility in mice: involvement of proinflammatory factors: nitric oxide and prostaglandins. Neuroscience 122, 1073–1080 (2003).
Galic, M. A. et al. Postnatal inflammation increases seizure susceptibility in adult rats. J. Neurosci. 28, 6904–6913 (2008).
Auvin, S. et al. Inflammation in rat pups subjected to short hyperthermic seizures enhances brain long-term excitability. Epilepsy Res. 86, 124–130 (2009).
Auvin, S., Mazarati, A. Shin, D. & Sankar, R. Inflammation enhances epileptogenesis in the developing rat brain. Neurobiol. Dis. 40, 303–310 (2010).
Kovacs, Z. et al. Facilitation of spike–wave discharge activity by lipopolysaccharides in Wistar Albino Glaxo/Rijswijk rats. Neuroscience 140, 731–742 (2006).
Mouihate, A. et al. Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J. Neurosci. 30, 7975–7983 (2010).
Harre, E. M., Galic, M. A., Mouihate, A., Noorbakhsh, F. & Pittman, Q. J. Neonatal inflammation produces selective behavioral deficits and alters N-methyl-D-aspartate receptor subunit mRNA in the adult rat brain. Eur. J. Neurosci. 27, 644–653 (2008).
Maroso, M. et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 16, 413–419 (2010).
Buckmaster, P. S. & Dudek, F. E. Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J. Neurophysiol. 77, 2685–2696 (1997).
Rizzi, M. et al. Glia activation and cytokine increase in rat hippocampus by kainic acid-induced status epilepticus during postnatal development. Neurobiol Dis. 14, 494–503 (2003).
Ravizza, T. & Vezzani, A. Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neuroscience 137, 301–308 (2006).
Plata-Salaman, C. R. et al. Kindling modulates the IL-1β system, TNF-α, TGF-β1, and neuropeptide mRNAs in specific brain regions. Brain Res. Mol. Brain Res. 75, 248–258 (2000).
Ravizza, T. et al. Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1β production. Neurobiol. Dis. 31, 327–333 (2008).
Longhi, L. et al. C1-inhibitor attenuates neurobehavioral deficits and reduces contusion volume after controlled cortical impact brain injury in mice. Crit. Care Med. 37, 659–665 (2009).
Clausen, F. et al. Neutralization of interleukin-1β modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 30, 385–396 (2009).
Lloyd, E., Somera-Molina, K., Van Eldik, L. J., Watterson, D. M. & Wainwright, M. S. Suppression of acute proinflammatory cytokine and chemokine upregulation by post-injury administration of a novel small molecule improves long-term neurologic outcome in a mouse model of traumatic brain injury. J. Neuroinflammation 5, 28 (2008).
Schwartz, M. & Shechter, R. Systemic inflammatory cells fight off neurodegenerative disease. Nat. Rev. Neurol. 6, 405–410 (2010).
Liesz, A. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat. Med. 15, 192–199 (2009).
Kelley, B. J. & Rodriguez, M. Seizures in patients with multiple sclerosis: epidemiology, pathophysiology and management. CNS Drugs 23, 805–815 (2009).
Perkins, N. D. Integrating cell-signaling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell. Biol. 8, 49–62 (2007).
Hoebe, K. & Beutler, B. Forward genetic analysis of TLR-signaling pathways: an evaluation. Adv. Drug Deliv. Rev. 60, 824–829 (2008).
Gilmore, T. D. Introduction to NF-κB: players, pathways, perspectives. Oncogene 25, 6680–6684 (2006).
O'Neill, L. A. & Kaltschmidt, C. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20, 252–258 (1997).
Pitkanen, A. & Lukasiuk, K. Molecular and cellular basis of epileptogenesis in symptomatic epilepsy. Epilepsy Behav. 14 (Suppl. 1), 16–25 (2009).
Balosso, S. et al. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1β. Brain 131, 3256–3265 (2008).
Viviani, B. et al. Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 23, 8692–8700 (2003).
Davis, C. N., Tabarean, I., Gaidarova, S., Behrens, M. M. & Bartfai, T. IL-1β induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J. Neurochem. 98, 1379–1389 (2006).
Sanchez-Alavez, M., Tabarean, I. V., Behrens, M. M. & Bartfai, T. Ceramide mediates the rapid phase of febrile response to IL-1β. Proc. Natl Acad. Sci. USA 103, 2904–2908 (2006).
Tabarean, I. V., Korn, H. & Bartfai, T. Interleukin-1β induces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Neuroscience 141, 1685–1695 (2006).
Zhang, R. et al. Acute p38-mediated inhibition of NMDA-induced outward currents in hippocampal CA1 neurons by interleukin-1β. Neurobiol. Dis. 38, 68–77 (2010).
Viviani, B., Gardoni, F. & Marinovich, M. Cytokines and neuronal ion channels in health and disease. Int. Rev. Neurobiol. 82, 247–263 (2007).
Bezzi, P. et al. CXCR4-activated astrocyte glutamate release via TNF-α: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 4, 702–710 (2001).
Hu, S., Sheng, W. S., Ehrlich, L. C., Peterson, P. K. & Chao, C. C. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 7, 153–159 (2000).
Stellwagen, D., Beattie, E. C., Seo, J. Y. & Malenka, R. C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. J. Neurosci. 25, 3219–3228 (2005).
Ferguson, A. R. et al. Cell death after spinal cord injury is exacerbated by rapid TNFα-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J. Neurosci. 28, 11391–11400 (2008).
Chen, C. & Bazan, N. G. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 77, 65–76 (2005).
Slanina, K. A. & Schweitzer, P. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacology 49, 653–659 (2005).
David, Y. et al. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J. Neurosci. 29, 10588–10599 (2009).
Cacheaux, L. P. et al. Transcriptome profiling reveals TGF-β signaling involvement in epileptogenesis. J. Neurosci. 29, 8927–8935 (2009).
Dantzer, R. Cytokine, sickness behavior, and depression. Neurol. Clin. 24, 441–460 (2006).
Cunningham, C. & Sanderson, D. J. Malaise in the water maze: untangling the effects of LPS and IL-1β on learning and memory. Brain Behav. Immun. 22, 1117–1127 (2008).
Bien, C. G. & Schramm, J. Treatment of Rasmussen encephalitis half a century after its initial description: promising prospects and a dilemma. Epilepsy Res. 86, 101–112 (2009).
Joels, M. & Baram, T. Z. The neuro-symphony of stress. Nat. Rev. Neurosci. 10, 459–466 (2009).
Brunson, K. L., Khan, N., Eghbal-Ahmadi, M. & Baram, T. Z. Corticotropin (ACTH) acts directly on amygdala neurons to downregulate corticotropin-releasing hormone gene expression. Ann. Neurol. 49, 304–312 (2001).
Baram, T. Z. & Hatalski, C. G. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 21, 471–476 (1998).
Pellock, J. M. et al. Infantile spasms: a U. S. consensus report. Epilepsia 51, 2175–2189 (2010).
Brunson, K. L., Avishai-Eliner, S. & Baram, T. Z. ACTH treatment of infantile spasms: mechanisms of its effects in modulation of neuronal excitability. Int. Rev. Neurobiol. 49, 185–197 (2002).
Sinclair, D. B. Prednisone therapy in pediatric epilepsy. Pediatr. Neurol. 28, 194–198 (2003).
Mikati, M. A., Kurdi, R., El-Khoury, Z., Rahi, A. & Raad, W. Intravenous immunoglobulin therapy in intractable childhood epilepsy: open-label study and review of the literature. Epilepsy Behav. 17, 90–94 (2010).
Marescaux, C. et al. Landau–Kleffner syndrome: a pharmacologic study of five cases. Epilepsia 31, 768–777 (1990).
Villani, F. & Avanzini, G. The use of immunoglobulins in the treatment of human epilepsy. Neurol. Sci. 23 (Suppl. 1), S33–S37 (2002).
You, S. J., Jung, D. E., Kim, H. D., Lee, H. S. & Kang, H. C. Efficacy and prognosis of a short course of prednisolone therapy for pediatric epilepsy. Eur. J. Paediatr. Neurol. 12, 314–320 (2008).
Mikati, M. A., Saab, R., Fayad, M. N. & Choueiri, R. N. Efficacy of intravenous immunoglobulin in Landau–Kleffner syndrome. Pediatr. Neurol. 26, 298–300 (2002).
Verhelst, H. et al. Steroids in intractable childhood epilepsy: clinical experience and review of the literature. Seizure 14, 412–421 (2005).
Baram, T. Z. et al. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics 97, 375–379 (1996).
Mackay, M. T. et al. Practice parameter: medical treatment of infantile spasms: report of the American Academy of Neurology and the Child Neurology Society. Neurology 62, 1668–1681 (2004).
Lux, A. L. et al. The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicenter randomised trial. Lance Neurol. 4, 712–717 (2005).
Gayatri, N. A., Ferrie, C. D. & Cross, H. Corticosteroids including ACTH for childhood epilepsy other than epileptic spasms. Cochrane Database of Systematic Reviews, Issue 1. Art. No.: CD005222. doi:10.1002/14651858.CD005222.pub2 (2007).
Crow, A. R., Song, S., Semple, J. W., Freedman, J. & Lazarus, A. H. A role for IL-1 receptor antagonist or other cytokines in the acute therapeutic effects of IVIg? Blood 109, 155–158 (2007).
Cullingford, T. Peroxisome proliferator-activated receptor alpha and the ketogenic diet. Epilepsia 49 (Suppl. 8), 70–72 (2008).
ClinicalTrials.gov Study of VX-765 in subjects with treatment-resistant partial epilepsy [online], (2010).
Tsan, M. F. & Gao, B. Endogenous ligands of Toll-like receptors. J. Leukoc. Biol. 76, 514–519 (2004).
Shlosberg, D., Benifla, M., Kaufer, D. & Friedman, A. Blood–brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 6, 393–403 (2010).
Hawkins, B. T. & Davis, T. P. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 57, 173–185 (2005).
Utech, M., Mennigen, R. & Bruewer, M. Endocytosis and recycling of tight junction proteins in inflammation. J. Biomed. Biotechnol. 2010, 484987 (2010).
Gloor, S. M. et al. Molecular and cellular permeability control at the blood–brain barrier. Brain Res. Brain Res. Rev. 36, 258–264 (2001).
Acknowledgements
This work was supported by contributions to A. Vezzani from Fondazione Cariplo, Fondazione Monzino and Parents Against Childhood Epilepsy (P.A.C.E.), the NIH R37 NS35439 and American Epilepsy Society Research Initiative award given to T. Z. Baram, and a Shaw Family Initiative On Inflammation award given to J. French. We apologize to the many authors whose work was not cited because of space limitations.
Author information
Authors and Affiliations
Contributions
A. Vezzani, J. French, T. Bartfai and T. Z. Baram contributed equally to researching data for the article, discussion of content, writing, and reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A. Vezzani is a holder of intellectual property at Vertex Pharmaceuticals, and J. French has received research support from Vertex Pharmaceuticals. T. Z. Baram has received honoraria from Pfizer and Questcor Pharmaceuticals, and has also acted as a consultant for Questcor Pharmaceuticals. T. Bartfai declares no competing interests.
Supplementary information
Supplementary Table 1
Inflammatory mediators in human epilepsy (DOC 68 kb)
Supplementary Table 2
Inflammatory mediators in brain tissue from experimental models of seizures and epilepsy (DOC 63 kb)
Supplementary Table 3
Inflammation, seizures and epileptogenesis: representative data from immature and adult animal models (DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Vezzani, A., French, J., Bartfai, T. et al. The role of inflammation in epilepsy. Nat Rev Neurol 7, 31–40 (2011). https://doi.org/10.1038/nrneurol.2010.178
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2010.178
This article is cited by
-
Leukocyte differential gene expression prognostic value for high versus low seizure frequency in temporal lobe epilepsy
BMC Neurology (2024)
-
Interleukin-6-elicited chronic neuroinflammation may decrease survival but is not sufficient to drive disease progression in a mouse model of Leigh syndrome
Journal of Inflammation (2024)
-
mTOR and neuroinflammation in epilepsy: implications for disease progression and treatment
Nature Reviews Neuroscience (2024)
-
Interleukins in Epilepsy: Friend or Foe
Neuroscience Bulletin (2024)
-
Decoding Epileptic Seizures: Exploring In Vitro Approaches to Unravel Pathophysiology and Propel Future Therapeutic Breakthroughs
Biomedical Materials & Devices (2024)