Key Points
-
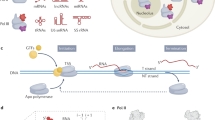
Maturation of pre-mRNA by capping, splicing, and cleavage and polyadenylation can occur co-transcriptionally at the gene; the substrate for these reactions is therefore a growing nascent RNA chain. Recent high-throughput sequencing of nascent RNA chains that are caught in the act of being synthesized shows that a large proportion of all splicing events occur co-transcriptionally.
-
Pre-mRNA processing is spatiotemporally coordinated with transcription by recruitment and kinetic coupling mechanisms, which facilitate binding of processing factors to the transcription elongation complex (TEC) and coordinate the rates of nascent RNA processing with transcription elongation.
-
Transcription elongation rate varies both between genes and between regions within a gene and can affect the outcomes of alternative splicing and alternative polyadenylation decisions. An important open question is whether elongation rate is regulated to effect changes in alternative mRNA processing.
-
Slow elongation lengthens the 'window of opportunity' for an upstream event to occur before it faces competition from a downstream RNA sequence element; conversely, fast elongation 'closes' that window of opportunity.
-
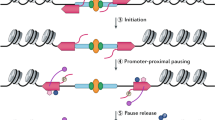
Recruitment of processing factors is mediated through contacts with the nascent RNA and protein surfaces on the TEC, most notably the carboxy-terminal domain (CTD) of RNA polymerase II that functions as a flexible and versatile 'landing pad'. Interfaces between the CTD and several binding proteins are known at atomic resolution.
-
Dynamic changes in the phosphorylation state of the CTD are synchronized with the cycle of transcription initiation, elongation and termination, and help to direct the traffic of processing factors on and off the landing pad.
-
Recent discoveries indicate a new class of regulators of mRNA production that target the kinetic coupling mechanism by influencing transcription elongation rate and splicing.
Abstract
Maturation of mRNA precursors often occurs simultaneously with their synthesis by RNA polymerase II (Pol II). The co-transcriptional nature of mRNA processing has permitted the evolution of coupling mechanisms that coordinate transcription with mRNA capping, splicing, editing and 3′ end formation. Recent experiments using sophisticated new methods for analysis of nascent RNA have provided important insights into the relative amount of co-transcriptional and post-transcriptional processing, the relationship between mRNA elongation and processing, and the role of the Pol II carboxy-terminal domain (CTD) in regulating these processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Beyer, A. L. & Osheim, Y. N. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 2, 754–765 (1988). This is an early graphic demonstration of co-transcriptional splicing.
Bird, G., Zorio, D. A. & Bentley, D. L. RNA polymerase II carboxy-terminal domain phosphorylation is required for cotranscriptional pre-mRNA splicing and 3′-end formation. Mol. Cell. Biol. 24, 8963–8969 (2004).
Kolasinska-Zwierz, P. et al. Differential chromatin marking of introns and expressed exons by H3K36me3. Nature Genet. 41, 376–381 (2009). This paper is the first to show that histone modifications correlate with splicing activity.
Bieberstein, N. I., Carrillo Oesterreich, F., Straube, K. & Neugebauer, K. M. First exon length controls active chromatin signatures and transcription. Cell Rep. 2, 62–68 (2012).
Gomez Acuna, L. I., Fiszbein, A., Allo, M., Schor, I. E. & Kornblihtt, A. R. Connections between chromatin signatures and splicing. Wiley Interdiscip. Rev. RNA 4, 77–91 (2013).
Berg, M. G. et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell 150, 53–64 (2012). This study shows a powerful effect of the U1 snRNA, which is a subunit of the spliceosome, in repressing premature polyadenylation and potentially regulating alternative poly(A) site choice.
Cramer, P., Pesce, C., Baralle, F. & Kornblihtt, A. Functional association between promoter structure and transcripts alternative splicing. Proc. Natl Acad. Sci. USA 94, 11456–11460 (1997). This paper provides early key evidence that transcription and splicing are mechanistically coupled.
Chiu, Y. L. et al. Tat stimulates cotranscriptional capping of HIV mRNA. Mol. Cell 10, 585–597 (2002).
Monsalve, M. et al. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol. Cell 6, 307–316 (2000).
Huang, Y. et al. Mediator complex regulates alternative mRNA processing via the MED23 subunit. Mol. Cell 45, 459–469 (2012).
Benson, M. J. et al. Heterogeneous nuclear ribonucleoprotein L-like (hnRNPLL) and elongation factor, RNA polymerase II, 2 (ELL2) are regulators of mRNA processing in plasma cells. Proc. Natl Acad. Sci. USA 109, 16252–16257 (2012).
Rosonina, E., Bakowski, M. A., McCracken, S. & Blencowe, B. J. Transcriptional activators control splicing and 3′-end cleavage levels. J. Biol. Chem. 278, 43034–43040 (2003).
Nagaike, T. et al. Transcriptional activators enhance polyadenylation of mRNA precursors. Mol. Cell 41, 409–418 (2011).
Schroeder, S. C., Zorio, D. A., Schwer, B., Shuman, S. & Bentley, D. A function of yeast mRNA cap methyltransferase, Abd1, in transcription by RNA polymerase II. Mol. Cell 13, 377–387 (2004).
Lenasi, T., Peterlin, B. M. & Barboric, M. Cap-binding protein complex links pre-mRNA capping to transcription elongation and alternative splicing through positive transcription elongation factor b (P-TEFb). J. Biol. Chem. 286, 22758–22768 (2011).
Martins, S. B. et al. Spliceosome assembly is coupled to RNA polymerase II dynamics at the 3′ end of human genes. Nature Struct. Mol. Biol. 18, 1115–1123 (2011).
Andersen, P. K., Lykke-Andersen, S. & Jensen, T. H. Promoter-proximal polyadenylation sites reduce transcription activity. Genes Dev. 26, 2169–2179 (2012).
Ji, X. et al. SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase. Cell 153, 855–868 (2013).
Sisodia, S. S., Sollner, W. B. & Cleveland, D. W. Specificity of RNA maturation pathways: RNAs transcribed by RNA polymerase III are not substrates for splicing or polyadenylation. Mol. Cell. Biol. 7, 3602–3612 (1987).
Smale, S. T. & Tjian, R. Transcription of herpes simplex virus tk sequences under the control of wild-type and mutant human RNA polymerase I promoters. Mol. Cell. Biol. 5, 352–362 (1985).
Corden, J. L., Cadena, D. L., Ahearn, J. M. Jr & Dahmus, M. E. A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II. Proc. Natl Acad. Sci. USA 82, 7934–7938 (1985).
Greenleaf, A. L. Positive patches and negative noodles: linking RNA processing to transcription? Trends Biochem. Sci. 18, 117–119 (1993). This paper presents a prescient speculation that the CTD functions in pre-mRNA processing.
Perales, R. & Bentley, D. “Cotranscriptionality”: the transcription elongation complex as a nexus for nuclear transactions. Mol. Cell 36, 178–191 (2009).
Egloff, S., Dienstbier, M. & Murphy, S. Updating the RNA polymerase CTD code: adding gene-specific layers. Trends Genet. 28, 333–341 (2012).
Hsin, J. P. & Manley, J. L. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev. 26, 2119–2137 (2012).
Ho, C. & Shuman, S. Distinct effector roles for Ser2 and Ser5 phosphorylation of the RNA polymerase II CTD in the recruitment and allosteric activation of mammalian capping enzyme. Mol. Cell 3, 405–411 (1999).
Johnson, S. A., Kim, H., Erickson, B. & Bentley, D. L. The export factor Yra1 modulates mRNA 3′ end processing. Nature Struct. Mol. Biol. 18, 1164–1171 (2011).
Lunde, B. M. et al. Cooperative interaction of transcription termination factors with the RNA polymerase II C-terminal domain. Nature Struct. Mol. Biol. 17, 1195–1201 (2010).
Hirose, Y. & Manley, J. L. RNA polymerase II is an essential mRNA polyadenylation factor. Nature 395, 93–96 (1998).
Hirose, Y., Tacke, R. & Manley, J. L. Phosphorylated RNA polymerase II stimulates pre-mRNA splicing. Genes Dev. 13, 1234–1239 (1999).
McCracken, S. et al. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature 385, 357–361 (1997). References 29–31 show the importance of the Pol II CTD for mRNA processing both in vitro and in vivo.
Rigo, F., Kazerouninia, A., Nag, A. & Martinson, H. G. The RNA tether from the poly(A) signal to the polymerase mediates coupling of transcription to cleavage and polyadenylation. Mol. Cell 20, 733–745 (2005).
Bird, G., Fong, N., Gatlin, J. C., Farabaugh, S. & Bentley, D. L. Ribozyme cleavage reveals connections between mRNA release from the site of transcription and pre-mRNA processing. Mol. Cell 20, 747–758 (2005).
Buratowski, S. Progression through the RNA polymerase II CTD cycle. Mol. Cell 36, 541–546 (2009).
Dujardin, G. et al. Transcriptional elongation and alternative splicing. Biochim. Biophys. Acta 1829, 134–140 (2013).
Nilsen, T. W. & Graveley, B. R. Expansion of the eukaryotic proteome by alternative splicing. Nature 463, 457–463 (2010).
Aebi, M., Hornig, H., Padgett, R. A., Reiser, J. & Weissmann, C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell 47, 555–565 (1986).
de la Mata, M. et al. A slow RNA polymerase II affects alternative splicing in vivo. Mol. Cell 12, 525–532 (2003). This study proposes the influential window of opportunity model to explain how transcription elongation rate could affect the outcome of alternative splicing decisions.
Rasmussen, E. B. & Lis, J. T. In vivo transcriptional pausing and cap formation on three Drosophila heat shock genes. Proc. Natl Acad. Sci. USA 90, 7923–7927 (1993).
Jiao, X. et al. Identification of a quality-control mechanism for mRNA 5′-end capping. Nature 467, 608–611 (2010).
Chang, J. H. et al. Dxo1 is a new type of eukaryotic enzyme with both decapping and 5′-3′ exoribonuclease activity. Nature Struct. Mol. Biol. 19, 1011–1017 (2012).
Jiao, X., Chang, J. H., Kilic, T., Tong, L. & Kiledjian, M. A mammalian pre-mRNA 5′ end capping quality control mechanism and an unexpected link of capping to pre-mRNA processing. Mol. Cell 50, 104–115 (2013). References 40–42 show a conserved function that monitors the quality of mRNA 5′ cap structures and that destroys transcripts that do not meet quality control standards.
Otsuka, Y., Kedersha, N. L. & Schoenberg, D. R. Identification of a cytoplasmic complex that adds a cap onto 5′-monophosphate RNA. Mol. Cell. Biol. 29, 2155–2167 (2009).
Fejes-Toth, K. et al. Post-transcriptional processing generates a diversity of 5′-modified long and short RNAs. Nature 457, 1028–1032 (2009).
Fernandez-Sanchez, M. E., Gonatopoulos-Pournatzis, T., Preston, G., Lawlor, M. A. & Cowling, V. H. S-adenosyl homocysteine hydrolase is required for Myc-induced mRNA cap methylation, protein synthesis, and cell proliferation. Mol. Cell. Biol. 29, 6182–6191 (2009).
Brannan, K. et al. mRNA decapping factors and the exonuclease Xrn2 function in widespread premature termination of RNA polymerase II transcription. Mol. Cell 46, 311–324 (2012).
Davidson, L., Kerr, A. & West, S. Co-transcriptional degradation of aberrant pre-mRNA by Xrn2. EMBO J. 31, 2566–2578 (2012). References 46 and 47 suggest that capping, which is a co-transcriptional mRNA processing step, can be reversed by decapping, which leads to premature transcriptional termination.
McCracken, S. et al. 5′-capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 11, 3306–3318 (1997).
Cho, E. J., Takagi, T. & Moore, C. R. and Buratowski, S. mRNA capping enzyme is recruited to the transcription complex by phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 11, 3319–3326 (1997). References 26, 48 and 49 show recruitment of mRNA-capping enzyme to the Pol II CTD and consequent allosteric activation of the guanylyltransferase.
Glover-Cutter, K., Kim, S., Espinosa, J. & Bentley, D. L. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nature Struct. Mol. Biol. 15, 71–78 (2008).
Listerman, I., Sapra, A. K. & Neugebauer, K. M. Cotranscriptional coupling of splicing factor recruitment and precursor messenger RNA splicing in mammalian cells. Nature Struct. Mol. Biol. 13, 815–822 (2006).
Wen, Y. & Shatkin, A. J. Transcription elongation factor hSPT5 stimulates mRNA capping. Genes Dev. 13, 1774–1779 (1999).
Mandal, S. S. et al. Functional interactions of RNA-capping enzyme with factors that positively and negatively regulate promoter escape by RNA polymerase II. Proc. Natl Acad. Sci. USA 101, 7572–7577 (2004).
Pei, Y. & Shuman, S. Interactions between fission yeast mRNA capping enzymes and elongation factor Spt5. J. Biol. Chem. 277, 19639–19648 (2002).
Schmidt, U. et al. Real-time imaging of cotranscriptional splicing reveals a kinetic model that reduces noise: implications for alternative splicing regulation. J. Cell Biol. 193, 819–829 (2011).
Audibert, A., Weil, D. & Dautry, F. In vivo kinetics of mRNA splicing and transport in mammalian cells. Mol. Cell. Biol. 22, 6706–6718 (2002).
Singh, J. & Padgett, R. A. Rates of in situ transcription and splicing in large human genes. Nature Struct. Mol. Biol. 16, 1128–1133 (2009).
Danko, C. G. et al. Signaling pathways differentially affect RNA polymerase II initiation, pausing, and elongation rate in cells. Mol. Cell 50, 212–222 (2013). This study uses powerful global run-on sequencing analysis to measure transcription elongation rates on many gene in vivo and shows remarkable variation in Pol II speed between genes and between 5′ and 3′ regions within genes.
Darzacq, X. et al. In vivo dynamics of RNA polymerase II transcription. Nature Struct. Mol. Biol. 14, 796–806 (2007).
Boireau, S. et al. The transcriptional cycle of HIV-1 in real-time and live cells. J. Cell Biol. 179, 291–304 (2007).
Bauren, G. & Wieslander, L. Splicing of Balbiani ring 1 gene pre-mRNA occurs simultaneously with transcription. Cell 76, 183–192 (1994).
Morlando, M. et al. Primary microRNA transcripts are processed co-transcriptionally. Nature Struct. Mol. Biol. 15, 902–909 (2008).
Darnell, J. E. Jr. Reflections on the history of pre-mRNA processing and highlights of current knowledge: a unified picture. RNA 19, 443–460 (2013).
Brugiolo, M., Herzel, L. & Neugebauer, K. M. Counting on co-transcriptional splicing. F1000Prime Rep. 5, 9 (2013).
Wuarin, J. & Schibler, U. Physical isolation of nascent RNA chains transcribed by RNA polymerase II: evidence for cotranscriptional splicing. Mol. Cell. Biol. 14, 7219–7225 (1994).
Bhatt, D. M. et al. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell 150, 279–290 (2012).
Pandya-Jones, A. et al. Splicing kinetics and transcript release from the chromatin compartment limit the rate of Lipid A-induced gene expression. RNA 19, 811–827 (2013).
Mili, S. & Steitz, J. A. Evidence for reassociation of RNA-binding proteins after cell lysis: implications for the interpretation of immunoprecipitation analyses. RNA 10, 1692–1694 (2004).
Khodor, Y. L., Menet, J. S., Tolan, M. & Rosbash, M. Cotranscriptional splicing efficiency differs dramatically between Drosophila and mouse. RNA 18, 2174–2186 (2012).
Khodor, Y. L. et al. Nascent-seq indicates widespread cotranscriptional pre-mRNA splicing in Drosophila. Genes Dev. 25, 2502–2512 (2011).
Tilgner, H. et al. Deep sequencing of subcellular RNA fractions shows splicing to be predominantly co-transcriptional in the human genome but inefficient for lncRNAs. Genome Res. 22, 1616–1625 (2012).
Churchman, L. S. & Weissman, J. S. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469, 368–373 (2011).
Carrillo Oesterreich, F., Preibisch, S. & Neugebauer, K. M. Global analysis of nascent RNA reveals transcriptional pausing in terminal exons. Mol. Cell 40, 571–581 (2010).
Ameur, A. et al. Total RNA sequencing reveals nascent transcription and widespread co-transcriptional splicing in the human brain. Nature Struct. Mol. Biol. 18, 1435–1440 (2011).
Windhager, L. et al. Ultrashort and progressive 4sU-tagging reveals key characteristics of RNA processing at nucleotide resolution. Genome Res. 22, 2031–2042 (2012). References 69–75 show that co-transcriptional splicing is widespread in vivo using genome-wide sequencing of nascent RNA populations.
Girard, C. et al. Post-transcriptional spliceosomes are retained in nuclear speckles until splicing completion. Nature Commun. 3, 994 (2012).
Hao, S. & Baltimore, D. RNA splicing regulates the temporal order of TNF-induced gene expression. Proc. Natl Acad. Sci. USA 110, 11934–11939 (2013).
Takashima, Y., Ohtsuka, T., Gonzalez, A., Miyachi, H. & Kageyama, R. Intronic delay is essential for oscillatory expression in the segmentation clock. Proc. Natl Acad. Sci. USA 108, 3300–3305 (2011).
Pandya-Jones, A. & Black, D. L. Co-transcriptional splicing of constitutive and alternative exons. RNA 15, 1896–1908 (2009).
Vargas, D. Y. et al. Single-molecule imaging of transcriptionally coupled and uncoupled splicing. Cell 147, 1054–1065 (2011).
Yeo, G. W., Van Nostrand, E., Holste, D., Poggio, T. & Burge, C. B. Identification and analysis of alternative splicing events conserved in human and mouse. Proc. Natl Acad. Sci. USA 102, 2850–2855 (2005).
Licatalosi, D. D. & Darnell, R. B. RNA processing and its regulation: global insights into biological networks. Nature Rev. Genet. 11, 75–87 (2010).
de la Mata, M., Lafaille, C. & Kornblihtt, A. R. First come, first served revisited: factors affecting the same alternative splicing event have different effects on the relative rates of intron removal. RNA 16, 904–912 (2010).
Abovich, N. & Rosbash, M. Cross-intron bridging interactions in the yeast commitment complex are conserved in mammals. Cell 89, 403–412 (1997).
Huranova, M. et al. The differential interaction of snRNPs with pre-mRNA reveals splicing kinetics in living cells. J. Cell Biol. 191, 75–86 (2010).
Hoskins, A. A. et al. Ordered and dynamic assembly of single spliceosomes. Science 331, 1289–1295 (2011).
Abelson, J. et al. Conformational dynamics of single pre-mRNA molecules during in vitro splicing. Nature Struct. Mol. Biol. 17, 504–512 (2010).
Braberg, H. et al. From structure to systems: high-resolution, quantitative genetic analysis of RNA polymerase II. Cell 154, 775–788 (2013).
Aguilera, A. & Garcia-Muse, T. R loops: from transcription byproducts to threats to genome stability. Mol. Cell 46, 115–124 (2012).
Lin, S., Coutinho-Mansfield, G., Wang, D., Pandit, S. & Fu, X. D. The splicing factor SC35 has an active role in transcriptional elongation. Nature Struct. Mol. Biol. 15, 819–826 (2008).
Close, P. et al. DBIRD complex integrates alternative mRNA splicing with RNA polymerase II transcript elongation. Nature 484, 386–389 (2012).
Batsche, E., Yaniv, M. & Muchardt, C. The human SWI/SNF subunit Brm is a regulator of alternative splicing. Nature Struct. Mol. Biol. 13, 22–29 (2006).
Alexander, R. D., Innocente, S. A., Barrass, J. D. & Beggs, J. D. Splicing-dependent RNA polymerase pausing in yeast. Mol. Cell 40, 582–593 (2010).
Zhou, H. L. et al. Hu proteins regulate alternative splicing by inducing localized histone hyperacetylation in an RNA-dependent manner. Proc. Natl Acad. Sci. USA 108, E627–E635 (2011).
Schor, I. E., Rascovan, N., Pelisch, F., Allo, M. & Kornblihtt, A. R. Neuronal cell depolarization induces intragenic chromatin modifications affecting NCAM alternative splicing. Proc. Natl Acad. Sci. USA 106, 4325–4330 (2009).
Saint-Andre, V., Batsche, E., Rachez, C. & Muchardt, C. Histone H3 lysine 9 trimethylation and HP1γ favor inclusion of alternative exons. Nature Struct. Mol. Biol. 18, 337–344 (2011).
Brody, Y. et al. The in vivo kinetics of RNA polymerase II elongation during co-transcriptional splicing. PLoS Biol. 9, e1000573 (2011).
Shukla, S. et al. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature 479, 74–79 (2011).
Kwak, H., Fuda, N. J., Core, L. J. & Lis, J. T. Precise maps of RNA polymerase reveal how promoters direct initiation and pausing. Science 339, 950–953 (2013).
Schwartz, S., Meshorer, E. & Ast, G. Chromatin organization marks exon-intron structure. Nature Struct. Mol. Biol. 16, 990–995 (2009).
Bintu, L. et al. Nucleosomal elements that control the topography of the barrier to transcription. Cell 151, 738–749 (2012).
Gunderson, F. Q., Merkhofer, E. C. & Johnson, T. L. Dynamic histone acetylation is critical for cotranscriptional spliceosome assembly and spliceosomal rearrangements. Proc. Natl Acad. Sci. USA 108, 2004–2009 (2011).
Hilleren, P., McCarthy, T., Rosbash, M., Parker, R. & Jensen, T. H. Quality control of mRNA 3′-end processing is linked to the nuclear exosome. Nature 413, 538–542 (2001).
de Almeida, S. F., Garcia-Sacristan, A., Custodio, N. & Carmo-Fonseca, M. A link between nuclear RNA surveillance, the human exosome and RNA polymerase II transcriptional termination. Nucleic Acids Res. 38, 8015–8026 (2010).
Eberle, A. B. et al. Splice-site mutations cause Rrp6-mediated nuclear retention of the unspliced RNAs and transcriptional down-regulation of the splicing-defective genes. PLoS ONE 5, e11540 (2010).
Rodriguez, J., Menet, J. S. & Rosbash, M. Nascent-seq indicates widespread cotranscriptional RNA editing in Drosophila. Mol. Cell 47, 27–37 (2012).
Schwartz, J. C. et al. FUS binds the CTD of RNA polymerase II and regulates its phosphorylation at Ser2. Genes Dev. 26, 2690–2695 (2012).
Dichmann, D. S. & Harland, R. M. fus/TLS orchestrates splicing of developmental regulators during gastrulation. Genes Dev. 26, 1351–1363 (2012).
Ryman, K., Fong, N., Bratt, E., Bentley, D. L. & Ohman, M. The C-terminal domain of RNA Pol II helps ensure that editing precedes splicing of the GluR-B transcript. RNA 13, 1071–1078 (2007).
Kim, H. et al. Gene-specific RNA polymerase II phosphorylation and the CTD code. Nature Struct. Mol. Biol. 17, 1279–1286 (2010).
Mayer, A. et al. Uniform transitions of the general RNA polymerase II transcription complex. Nature Struct. Mol. Biol. 17, 1272–1278 (2010).
Zaret, K. S. & Sherman, F. DNA sequence required for efficient transcription termination in yeast. Cell 28, 563–573 (1982).
Logan, J., Falck, P. E., Darnell, J. E. J. & Shenk, T. A poly(A) addition site and a downstream termination region are required for efficient cessation of transcription by RNA polymerase II in the mouse β maj-globin gene. Proc. Natl Acad. Sci. USA 84, 8306–8310 (1987).
Whitelaw, E. & Proudfoot, N. α-thalassaemia caused by a poly(A) site mutation reveals that transcriptional termination is linked to 3′ end processing in the human α2 globin gene. EMBO J. 5, 2915–2922 (1986).
Nojima, T., Dienstbier, M., Murphy, S., Proudfoot, N. J. & Dye, M. J. Definition of RNA polymerase II CoTC terminator elements in the human genome. Cell Rep. 3, 1080–1092 (2013).
Connelly, S. & Manley, J. L. A functional mRNA polyadenylation signal is required for transcription termination by RNA polymerase II. Genes Dev. 2, 440–452 (1988).
Niwa, M. & Berget, S. M. Mutation of the AAUAAA polyadenylation signal depresses in vitro splicing of proximal but not distal introns. Genes Dev. 5, 2086–2095 (1991).
Niwa, M., Rose, S. D. & Berget, S. M. In vitro polyadenylation is stimulated by the presence of an upstream intron. Genes Dev. 4, 1552–1559 (1990).
Lu, S. & Cullen, B. R. Analysis of the stimulatory effect of splicing on mRNA production and utilization in mammalian cells. RNA 9, 618–630 (2003).
Martinson, H. G. An active role for splicing in 3′-end formation. Wiley Interdiscip. Rev. RNA 2, 459–470 (2011).
David, C. J., Boyne, A. R., Millhouse, S. R. & Manley, J. L. The RNA polymerase II C-terminal domain promotes splicing activation through recruitment of a U2AF65–Prp19 complex. Genes Dev. 25, 972–983 (2011).
Venkataraman, K., Brown, K. M. & Gilmartin, G. M. Analysis of a noncanonical poly(A) site reveals a tripartite mechanism for vertebrate poly(A) site recognition. Genes Dev. 19, 1315–1327 (2005).
Bauren, G., Belikov, S. & Wieslander, L. Transcriptional termination in the Balbiani ring 1 gene is closely coupled to 3′-end formation and excision of the 3′-terminal intron. Genes Dev. 12, 2759–2769 (1998).
Di Giammartino, D. C., Nishida, K. & Manley, J. L. Mechanisms and consequences of alternative polyadenylation. Mol. Cell 43, 853–866 (2011).
Lianoglou, S., Garg, V., Yang, J. L., Leslie, C. S. & Mayr, C. Ubiquitously transcribed genes use alternative polyadenylation to achieve tissue-specific expression. Genes Dev. 27, 2380–2396 (2013).
Mayr, C. & Bartel, D. P. Widespread shortening of 3′ UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 138, 673–684 (2009).
Das, R. et al. SR proteins function in coupling RNAP II transcription to pre-mRNA splicing. Mol. Cell 26, 867–881 (2007).
Enriquez, H. P., Levitt, N., Briggs, D. & Proudfoot, N. J. A pause site for RNA polymerase II is associated with termination of transcription. EMBO J. 10, 1833–1842 (1991).
Peterson, M. L., Bertolino, S. & Davis, F. An RNA polymerase pause site is associated with the immunoglobulin mus poly(A) site. Mol. Cell. Biol. 22, 5606–5615 (2002).
Pinto, P. A. et al. RNA polymerase II kinetics in polo polyadenylation signal selection. EMBO J. 30, 2431–2444 (2011).
Hazelbaker, D. Z., Marquardt, S., Wlotzka, W. & Buratowski, S. Kinetic competition between RNA polymerase II and Sen1-dependent transcription termination. Mol. Cell 49, 55–66 (2013).
Eperon, L. P., Graham, I. R., Griffiths, A. D. & Eperon, I. C. Effects of RNA secondary structure on alternative splicing of pre-mRNA: is folding limited to a region behind the transcribing RNA polymerase? Cell 54, 393–401 (1988).
Zhao, P., Zhang, W. & Chen, S. J. Cotranscriptional folding kinetics of ribonucleic acid secondary structures. J. Chem. Phys. 135, 245101 (2011).
Pan, T. & Sosnick, T. RNA folding during transcription. Annu. Rev. Biophys. Biomol. Struct. 35, 161–175 (2006).
Meyer, M., Plass, M., Perez-Valle, J., Eyras, E. & Vilardell, J. Deciphering 3′ss selection in the yeast genome reveals an RNA thermosensor that mediates alternative splicing. Mol. Cell 43, 1033–1039 (2011).
Zamft, B., Bintu, L., Ishibashi, T. & Bustamante, C. Nascent RNA structure modulates the transcriptional dynamics of RNA polymerases. Proc. Natl Acad. Sci. USA 109, 8948–8953 (2012).
Egloff, S. & Murphy, S. Cracking the RNA polymerase II CTD code. Trends Genet. 24, 280–288 (2008).
Jasnovidova, O. & Stefl, R. The CTD code of RNA polymerase II: a structural view. Wiley Interdiscip. Rev. RNA 4, 1–16 (2013).
Tardiff, D. F., Lacadie, S. A. & Rosbash, M. A genome-wide analysis indicates that yeast pre-mRNA splicing is predominantly posttranscriptional. Mol. Cell 24, 917–929 (2006).
Spiluttini, B. et al. Splicing-independent recruitment of U1 snRNP to a transcription unit in living cells. J. Cell Sci. 123, 2085–2093 (2010).
Ujvari, A. & Luse, D. S. Newly initiated RNA encounters a factor involved in splicing immediately upon emerging from within RNA polymerase II. J. Biol. Chem. 279, 49773–49779 (2004).
Schroeder, S. C., Schwer, B., Shuman, S. & Bentley, D. Dynamic association of capping enzymes with transcribing RNA polymerase II. Genes Dev. 14, 2435–2440 (2000).
Komarnitsky, P., Cho, E. J. & Buratowski, S. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 14, 2452–2460 (2000). This study shows that dynamic changes in Pol II CTD phosphorylation are coordinated with transcription initiation, elongation and termination.
Vasiljeva, L., Kim, M., Mutschler, H., Buratowski, S. & Meinhart, A. The Nrd1–Nab3–Sen1 termination complex interacts with the Ser5-phosphorylated RNA polymerase II C-terminal domain. Nature Struct. Mol. Biol. 15, 795–804 (2008).
Licatalosi, D. D. et al. Functional interaction of yeast pre-mRNA 3′ end processing factors with RNA polymerase II. Mol. Cell 9, 1101–1111 (2002).
Buratowski, S. The CTD code. Nature Struct. Biol. 10, 679–680 (2003).
Gu, B., Eick, D. & Bensaude, O. CTD serine-2 plays a critical role in splicing and termination factor recruitment to RNA polymerase II in vivo. Nucleic Acids Res. 41, 1591–1603 (2013).
Munoz, M. J. et al. DNA damage regulates alternative splicing through inhibition of RNA polymerase II elongation. Cell 137, 708–720 (2009).
Schwer, B. & Shuman, S. Deciphering the RNA polymerase II CTD code in fission yeast. Mol. Cell 43, 311–318 (2011). This paper uses an elegant genetic test of the CTD code hypothesis to show that the essential function of Ser5 phosphorylation is to recruit the mRNA-capping enzyme.
Schwer, B., Sanchez, A. M. & Shuman, S. Punctuation and syntax of the RNA polymerase II CTD code in fission yeast. Proc. Natl Acad. Sci. USA 109, 18024–18029 (2012).
Lee, J. M. & Greenleaf, A. L. CTD kinase large subunit is encoded by CTK1, a gene required for normal growth of Saccharomyces cerevisiae. Gene Expr. 1, 149–167 (1991).
Ahn, S. H., Kim, M. & Buratowski, S. Phosphorylation of serine 2 within the RNA polymerase II C-terminal domain couples transcription and 3′ end processing. Mol. Cell 13, 67–76 (2004).
Shen, Z., St-Denis, A. & Chartrand, P. Cotranscriptional recruitment of She2p by RNA Pol II elongation factor Spt4–Spt5/DSIF promotes mRNA localization to the yeast bud. Genes Dev. 24, 1914–1926 (2010).
Suh, M. H. et al. A dual interface determines the recognition of RNA polymerase II by RNA capping enzyme. J. Biol. Chem. 285, 34027–34038 (2010).
Luco, R. F. et al. Regulation of alternative splicing by histone modifications. Science 327, 996–1000 (2010).
Pradeepa, M. M., Sutherland, H. G., Ule, J., Grimes, G. R. & Bickmore, W. A. Psip1/Ledgf p52 binds methylated histone H3K36 and splicing factors and contributes to the regulation of alternative splicing. PLoS Genet. 8, e1002717 (2012).
Sims, R. J. 3rd et al. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol. Cell 28, 665–676 (2007).
Govind, C. K. et al. Phosphorylated Pol II CTD recruits multiple HDACs, including Rpd3C(S), for methylation-dependent deacetylation of ORF nucleosomes. Mol. Cell 39, 234–246 (2010).
Kizer, K. O. et al. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol. Cell. Biol. 25, 3305–3316 (2005).
de Almeida, S. F. et al. Splicing enhances recruitment of methyltransferase HYPB/Setd2 and methylation of histone H3 Lys36. Nature Struct. Mol. Biol. 18, 977–983 (2011).
Kim, S., Kim, H., Fong, N., Erickson, B. & Bentley, D. L. Pre-mRNA splicing is a determinant of histone H3K36 methylation. Proc. Natl Acad. Sci. USA 108, 13564–13569 (2011).
Lionnet, T. & Singer, R. H. Transcription goes digital. EMBO Rep. 13, 313–321 (2012).
Batada, N. N., Westover, K. D., Bushnell, D. A., Levitt, M. & Kornberg, R. D. Diffusion of nucleoside triphosphates and role of the entry site to the RNA polymerase II active center. Proc. Natl Acad. Sci. USA 101, 17361–17364 (2004).
Ehrensberger, A. H., Kelly, G. P. & Svejstrup, J. Q. Mechanistic interpretation of promoter-proximal peaks and RNAPII density maps. Cell 154, 713–715 (2013).
Zhou, Q., Li, T. & Price, D. H. RNA polymerase II elongation control. Annu. Rev. Biochem. 81, 119–143 (2012).
Core, L. J., Waterfall, J. J. & Lis, J. T. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 (2008).
Larson, D. R., Zenklusen, D., Wu, B., Chao, J. A. & Singer, R. H. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science 332, 475–478 (2011).
Payne, J. M., Laybourn, P. J. & Dahmus, M. E. The transition of RNA polymerase II from initiation to elongation is associated with phosphorylation of the carboxyl-terminal domain of subunit IIA. J. Biol. Chem. 264, 19621–19629 (1989).
Heidemann, M., Hintermair, C., Voss, K. & Eick, D. Dynamic phosphorylation patterns of RNA polymerase II CTD during transcription. Biochim. Biophys. Acta 1829, 55–62 (2013).
Ranuncolo, S. M., Ghosh, S., Hanover, J. A., Hart, G. W. & Lewis, B. A. Evidence of the involvement of O-GlcNAc-modified human RNA polymerase II CTD in transcription in vitro and in vivo. J. Biol. Chem. 287, 23549–23561 (2012).
Mayer, A. et al. CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science 336, 1723–1725 (2012).
Garrido-Lecca, A. & Blumenthal, T. RNA polymerase II C-terminal domain phosphorylation patterns in Caenorhabditis elegans operons, polycistronic gene clusters with only one promoter. Mol. Cell. Biol. 30, 3887–3893 (2010).
Bartkowiak, B. et al. CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev. 24, 2303–2316 (2010).
Bitoun, E., Oliver, P. L. & Davies, K. E. The mixed-lineage leukemia fusion partner AF4 stimulates RNA polymerase II transcriptional elongation and mediates coordinated chromatin remodeling. Hum. Mol. Genet. 16, 92–106 (2007).
Lin, C. et al. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol. Cell 37, 429–437 (2010).
Roy, R. et al. The MO15 cell-cycle kinase is associated with the TFIIH transcription DNA-repair factor. Cell 79, 1093–1101 (1994).
Akhtar, M. S. et al. TFIIH kinase places bivalent marks on the carboxy-terminal domain of RNA polymerase II. Mol. Cell 34, 387–393 (2009).
Czudnochowski, N., Bosken, C. A. & Geyer, M. Serine-7 but not serine-5 phosphorylation primes RNA polymerase II CTD for P-TEFb recognition. Nature Commun. 3, 842 (2012).
He, X. et al. Functional interactions between the transcription and mRNA 3′ end processing machineries mediated by Ssu72 and Sub1. Genes Dev. 17, 1030–1042 (2003).
Xiang, K. et al. Crystal structure of the human symplekin-Ssu72-CTD phosphopeptide complex. Nature 467, 729–733 (2010).
Phatnani, H. P., Jones, J. C. & Greenleaf, A. L. Expanding the functional repertoire of CTD kinase I and RNA polymerase II: novel phosphoCTD-associating proteins in the yeast proteome. Biochemistry 43, 15702–15719 (2004).
Ghosh, A., Shuman, S. & Lima, C. D. Structural insights to how mammalian capping enzyme reads the CTD code. Mol. Cell 43, 299–310 (2011).
Meinhart, A. & Cramer, P. Recognition of RNA polymerase II carboxy-terminal domain by 3′-RNA-processing factors. Nature 430, 223–226 (2004).
Kubicek, K. et al. Serine phosphorylation and proline isomerization in RNAP II CTD control recruitment of Nrd1. Genes Dev. 26, 1891–1896 (2012).
Kim, M. et al. The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 432, 517–522 (2004).
Egloff, S. et al. Serine-7 of the RNA polymerase II CTD is specifically required for snRNA gene expression. Science 318, 1777–1779 (2007).
Chen, H.-C. & Cheng, S.-C. Functional roles of protein splicing factors. Biosci. Rep. 32, 345–359 (2012).
Cramer, P., Bushnell, D. A. & Kornberg, R. D. Structural basis of transcription: RNA polymerase II at 2.8 angstrom resolution. Science 292, 1863–1876 (2001).
Acknowledgements
The author apologizes to colleagues whose work was not referenced because of space limitations. Work in D.L.B's laboratory is supported by the US National Institutes of Health grants GM58163 and GM063873. The author thanks Y. Shav-Tal, D. Licatalosi, T. Blumenthal, R. Davis, D. Ish-Horowitz and members of his laboratory for discussions, and K. A. Nasmyth, J. Cooper and Cancer Research UK for their hospitality during preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- Carboxy-terminal domain
-
(CTD). Located in the large subunit of RNA polymerase II, the CTD is a signature feature of this polymerase that contains conserved heptad repeats (52 YSPTSPS repeats in humans) and is not found in RNA polymerases I and III.
- Transcription elongation complex
-
(TEC). A complex of RNA polymerase that is stably bound to the DNA template, polymerase-bound proteins and the nascent RNA chain.
- Alternative splicing
-
The most important mechanism by which the transcriptome is diversified through production of multiple mRNAs from a single gene. Alternatively spliced mRNAs differ in their coding and non-coding sequences as a result of selective inclusion and exclusion of exon and intron sequences.
- Transcription start site
-
The site on the DNA where the first phosphodiester bond in a RNA transcript is formed.
- Commitment complex
-
A stable complex formed between an intron-containing pre-mRNA, the U1 small nuclear ribonucleoprotein particle bound to the 5′ splice site, branch point-binding protein bound to the branch point and U2 auxiliary factor bound to the 3′ splice site. Once formed, it remains committed to the completion of splicing even when challenged with an excess of pre-mRNA substrate.
- R-loops
-
Structures that are formed by the hybridization of RNA transcripts to double-stranded DNA in which the displaced non-template DNA strands are looped out. Their recombinogenic nature causes genomic instability.
- Alternative polyadenylation
-
The decision to process at one of multiple poly(A) sites that are present at the 3′ ends of most human genes. This decision can have important functional consequences because it determines the sequence content of the 3′ untranslated region, which controls mRNA stability and translational efficiency.
Rights and permissions
About this article
Cite this article
Bentley, D. Coupling mRNA processing with transcription in time and space. Nat Rev Genet 15, 163–175 (2014). https://doi.org/10.1038/nrg3662
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3662
This article is cited by
-
ASAPA: a bioinformatic pipeline based on Iso-Seq that identifies the links among alternative splicing, alternative transcription initiation and alternative polyadenylation
Functional & Integrative Genomics (2024)
-
PAPγ associates with PAXT nuclear exosome to control the abundance of PROMPT ncRNAs
Nature Communications (2023)
-
RBP–RNA interactions in the control of autoimmunity and autoinflammation
Cell Research (2023)
-
The conserved RNA-binding protein Seb1 promotes cotranscriptional ribosomal RNA processing by controlling RNA polymerase I progression
Nature Communications (2023)
-
An unnatural enzyme with endonuclease activity towards small non-coding RNAs
Nature Communications (2023)