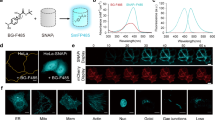
Abstract
A number of photoactivatable GFP-like fluorescent proteins (PAFPs) have been reported whose fluorescence can be switched on or whose fluorescent state can be modified by relatively intense irradiation at a specific wavelength. The use of these proteins gives unique opportunities to photolabel and track fusion proteins in a living cell. Here, we provide a protocol for the primary visualization, photoactivation and tracking of two monomeric PAFPs recently developed in our lab. Both these proteins, PS-CFP2 and Dendra2, are fluorescent and can be visualized before photoactivation. Upon photoactivation, their excitation and emission spectra undergo a dramatic red shift. The brightness of their initial and photoconverted states, along with the high dynamic ranges of both proteins, make them an attractive tool for protein photolabeling. Excluding genetic constructs cloning, cell culturing and transfection, the whole protocol may take anywhere from 10 min to several hours, depending on motility of the protein being studied.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lukyanov, K.A., Chudakov, D.M., Lukyanov, S. & Verkhusha, V.V. Innovation: photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell Biol. 6, 885–891 (2005).
Patterson, G.H. & Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 (2002).
Chudakov, D.M. et al. Photoswitchable cyan fluorescent protein for protein tracking. Nat. Biotechnol. 22, 1435–1439 (2004).
van Thor, J.J., Gensch, T., Hellingwerf, K.J. & Johnson, L.N. Phototransformation of green fluorescent protein with UV and visible light leads to decarboxylation of glutamate 222. Nat. Struct. Biol. 9, 37–41 (2002).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 12651–12656 (2002).
Wiedenmann, J. et al. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. USA 101, 15905–15910 (2004).
Tsutsui, H., Karasawa, S., Shimizu, H., Nukina, N. & Miyawaki, A. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 6, 233–238 (2005).
Gurskaya, N.G. et al. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 24, 461–465 (2006).
Mizuno, H. et al. Photo-induced peptide cleavage in the green-to-red conversion of a fluorescent protein. Mol. Cell 12, 1051–1058 (2003).
Lukyanov, K.A. et al. Natural animal coloration can be determined by a nonfluorescent green fluorescent protein homolog. J. Biol. Chem. 275, 25879–25882 (2000).
Chudakov, D.M., Feofanov, A.V., Mudrik, N.N., Lukyanov, S. & Lukyanov, K.A. Chromophore environment provides clue to “kindling fluorescent protein” riddle. J. Biol. Chem. 278, 7215–7219 (2003).
Chudakov, D.M. et al. Kindling fluorescent proteins for precise in vivo photolabeling. Nat. Biotechnol. 21, 191–194 (2003).
Ando, R., Mizuno, H. & Miyawaki, A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science 306, 1370–1373 (2004).
Stiel, A.C. et al. 1.8 A bright-state structure of the reversibly switchable fluorescent protein Dronpa guides the generation of fast switching variants. Biochem. J. 402, 35–42 (2007).
Henderson, J.N., Ai, H.W., Campbell, R.E. & Remington, S.J. Structural basis for reversible photobleaching of a green fluorescent protein homologue. Proc. Natl. Acad. Sci. USA 104, 6672–6677 (2007).
Andresen, M. et al. Structure and mechanism of the reversible photoswitch of a fluorescent protein. Proc. Natl. Acad. Sci. USA 102, 13070–13074 (2005).
Andresen, M. et al. Structural basis for reversible photoswitching in Dronpa. Proc. Natl. Acad. Sci. USA Jul 23 (2007) [Epub ahead of print].
Zhang, L. et al. Method for real-time monitoring of protein degradation at the single cell level. Biotechniques 42, 446, 448, 450 (2007).
Hofmann, M., Eggeling, C., Jakobs, S. & Hell, S.W. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc. Natl. Acad. Sci. USA 102, 17565–17569 (2005).
Schwentker, M.A. et al. Wide-field subdiffraction RESOLFT microscopy using fluorescent protein photoswitching. Microsc. Res. Tech. 70, 269–280 (2007).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Chudakov, D.M., Lukyanov, S. & Lukyanov, K.A. Fluorescent proteins as a toolkit for in vivo imaging. Trends Biotechnol. 23, 605–613 (2005).
Xia, J., Kim, S.H., Macmillan, S. & Truant, R. Practical three color live cell imaging by widefield microscopy. Biol. Proced. Online 8, 63–68 (2006).
Post, J.N., Lidke, K.A., Rieger, B. & Arndt-Jovin, D.J. One- and two-photon photoactivation of a paGFP-fusion protein in live Drosophila embryos. FEBS Lett. 579, 325–330 (2005).
Stark, D.A. & Kulesa, P.M. An in vivo comparison of photoactivatable fluorescent proteins in an avian embryo model. Dev. Dyn. 236, 1583–1594 (2007).
PerkinElmer. BioTechniques Protocol Guide 41, 63 (2006).
Acknowledgements
We are grateful to Elena Filinova for the help. This work was supported by Grants from Molecular and Cell Biology Program RAS, EC FP-6 Integrated Project LSHG-CT-2003-503259, Howard Hughes Medical Institute grant HHMI 55005618 and from the National Institutes of Health (GM070358). D.M.C. and K.A.L. are supported by Grants of the President of Russian Federation MK-8236.2006.4 and Russian Science Support Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Authors have interest in Evrogen JSC, which commercializes PS-CFP2 and Dendra2.
Rights and permissions
About this article
Cite this article
Chudakov, D., Lukyanov, S. & Lukyanov, K. Tracking intracellular protein movements using photoswitchable fluorescent proteins PS-CFP2 and Dendra2. Nat Protoc 2, 2024–2032 (2007). https://doi.org/10.1038/nprot.2007.291
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.291
This article is cited by
-
Bridging live-cell imaging and next-generation cancer treatment
Nature Reviews Cancer (2023)
-
Optical pulse labeling studies reveal exogenous seeding slows α-synuclein clearance
npj Parkinson's Disease (2022)
-
Mechanisms of breast cancer metastasis
Clinical & Experimental Metastasis (2022)
-
Tracking distinct microglia subpopulations with photoconvertible Dendra2 in vivo
Journal of Neuroinflammation (2021)
-
Photodynamic studies reveal rapid formation and appreciable turnover of tau inclusions
Acta Neuropathologica (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.