Abstract
Development passes through sensitive periods, during which plasticity allows for genetic and environmental factors to exert indelible influence on the maturation of the organism. In the context of central nervous system development, such sensitive periods shape the formation of neurocircuits that mediate, regulate, and control behavior. This general mechanism allows for development to be guided by both the genetic blueprint as well as the environmental context. While allowing for adaptation, such sensitive periods are also vulnerability windows during which external and internal factors can confer risk to disorders by derailing otherwise resilient developmental programs. Here we review developmental periods that are sensitive to monoamine signaling and impact adult behaviors of relevance to psychiatry. Specifically, we review (1) a serotonin-sensitive period that impacts sensory system development, (2) a serotonin-sensitive period that impacts cognition, anxiety- and depression-related behaviors, and (3) a dopamine- and serotonin-sensitive period affecting aggression, impulsivity and behavioral response to psychostimulants. We discuss preclinical data to provide mechanistic insight, as well as epidemiological and clinical data to point out translational relevance. The field of translational developmental neuroscience has progressed exponentially providing solid conceptual advances and unprecedented mechanistic insight. With such knowledge at hand and important methodological innovation ongoing, the field is poised for breakthroughs elucidating the developmental origins of neuropsychiatric disorders, and thus understanding pathophysiology. Such knowledge of sensitive periods that determine the developmental trajectory of complex behaviors is a necessary step towards improving prevention and treatment approaches for neuropsychiatric disorders.
Similar content being viewed by others
INTRODUCTION
Neuronal activity during development shapes functional connectivity between neurons and thus determines the ‘wiring’ of the mammalian brain. As best described for the development of sensory systems, such plasticity is often restricted to specific developmental periods, so-called sensitive periods. If certain events must occur within specified time windows to allow for normal maturation, respective time windows are referred to as critical periods. The best-studied example is the critical period for the formation of ocular dominance columns, when retinal activity determines columnar size (Espinosa and Stryker, 2012; Hensch, 2005). Sensitive or critical period plasticity allows for circuit maturation to respond/adapt to external (environmental) and internal (genetic) factors. Although adaptive from an evolutionary perspective, heightened plasticity during sensitive periods also permits environmental and genetic factors to shift ontogenetic pathways and confer risk for disorders.
Here we review developmental periods that are sensitive to monoamine signaling and influence adult behavior of important relevance for psychiatry. First, we provide a short overview of dopamine (DA) and serotonin (5-HT) system development, because this information relates to mechanistic aspects of monoamine-sensitive periods. We then briefly review the murine neonatal 5-HT-sensitive period with consequences on sensory system development, because these findings provide the best-characterized examples of stark neuroanatomical malleability and reveal guiding conceptual principles that relate to monoamine-sensitive periods in general. Thereafter, we focus on two periods: the murine early postnatal period, highlighting its role in shaping adult anxiety/depression-related behaviors and cognition, and the murine periadolescent (PA) period, highlighting monoamine signaling-related consequences on adult aggression and behavioral stimulant sensitivity. For both periods, we review preclinical data to provide mechanistic insight, as well as epidemiological and clinical data to point out translational relevance. The murine embryonic period is reviewed by Stanwood et al in this issue.
MONOAMINE SYSTEM DEVELOPMENT
In humans, 5-HT neurons are first detected when the embryo is 5 weeks old (Sundstrom et al, 1993), with rapid growth and proliferation until the 10th week of gestation (Levallois et al, 1997). After 15 weeks of gestation, 5-HT cell bodies cluster in the raphe nuclei (Takahashi et al, 1986). Levels of 5-HT increase during the first 2 years and then decline to adult levels after the age of 5 years (Sodhi and Sanders-Bush, 2004). In rodents, this dynamic maturation of the 5-HT system is also present (Figure 1). The first 5-HT neurons appear at the 12th day of rodent gestation (Lauder and Bloom, 1974). 5-HTergic neurons start releasing 5-HT on embryonic day 13 (E13) (Lambe et al, 2000; Lidov and Molliver, 1982a), and levels of 5-HT peak within the first postnatal week, after which they decline, reaching adult levels at around postnatal day 15 (P15; Hohmann et al, 1988). 5-HTergic neurons continue to elaborate their innervation patterns throughout the embryonic and early postnatal life, until about P21 (Lauder, 1990). Hence, in mice and rats, the presynaptic 5-HT components surface around E12 and mature until about P21. An additional aspect of presynaptic 5-HTergic system maturation is the transient adoption of a 5-HTergic phenotype by several otherwise non-5-HTergic neuron populations during late embryonic and early postnatal development (Gaspar et al, 2003; Lebrand et al, 1996, 1998; Salichon et al, 2001). Lastly, 5-HT receptors are expressed early in embryonic development, even before 5-HTergic afferents reach their innervation targets (Bonnin et al, 2006; Hellendall et al, 1993). During these early developmental stages, 5-HT arising from placental sources acts on 5-HT heteroreceptors, enabling developmental 5-HT signaling even before 5-HTergic axons have reached their targets (Bonnin et al, 2011).
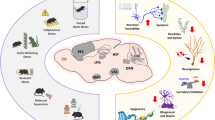
Transient peaks in monoamine system development. The graph displays relative levels of 5-HTergic and DAergic measures in the CNS across rat and mouse development: monoamine tissue concentration (5-HT of placental and raphe origin and DA), DA receptor binding (DA receptors) DAergic firing frequency (DAergic activity), and DAT binging (DAT). Green labels 5-HTergic aspects, and red labels DAergic aspects. The dashed line separates embryonic (left) from postnatal (right) development. ‘Transiently 5-HTergic’ demarks the time window during which non-5-HTergic neurons transiently adopt a 5-HTergic phenotype. CNS, cerebrospinal fluid; DA, dopamine; 5-HT, serotonin.
Midbrain DA neurons appear during the second month of gestation in humans, and between E12 and E15 in rodents (Olson and Seiger, 1972). In rats, starting at E15, DA-positive fibers pass through the developing striatum to cortical regions. The development of cortical DA innervation continues to increase until P60, after which density and topography of DAergic afferents remain constant (Kalsbeek et al, 1988). Interestingly, several measures of DAergic system maturation transiently peak during adolescence (Figure 1). For example, DA transporter (DAT) density in the striatum increases from P25 through P50, but then decreases continuously until P90 (Moll et al, 2000; Tarazi et al, 1998a). DA receptors are first expressed by E14 in the rat and E12 in the mouse (Araki et al, 2007; Jung and Bennett, 1996), and during postnatal development, striatal DA receptor-binding capacity continues to gradually increase until P28–P40, after which it diminishes again to reach stable levels at around P60 (Giorgi et al, 1987; Tarazi et al, 1998b; Teicher et al, 1995). Likewise, DAergic cell activity in mice is low at weaning, then increases to a peak at P45, after which it declines once again (McCutcheon and Marinelli, 2009). In the ventral tegmental area (VTA), this transient increase in DAergic activity is characterized by increased non-bursting activity and longer burst duration (McCutcheon et al, 2012). Lastly, tissue DA levels peak between P25 and P40 (Noisin and Thomas, 1988). Hence, pre- and postsynaptic DA system maturation follows an expansion–contraction course, peaking during late adolescence.
Due to the expression of the pre- and postsynaptic components of the 5-HT and DA systems during development, with monoaminergic neurons innervating and releasing neurotransmitter, and with extraneuronal sources providing central monoamines, it is easily conceivable that 5-HT and DA have a critical role in modulating neurodevelopment and functional circuit formation. Animal studies support this hypothesis, and have uncovered consequences of dysregulated developmental monoamine signaling on cytoarchitecture and -physiology, neuronal circuit properties, and behavior. Interestingly and counter intuitively, too much monoamine signaling seems to be more disruptive to normal development than too little.
A 5-HT-SENSITIVE DEVELOPMENTAL PERIOD IMPACTING THE SOMATOSENSORY AND VISUAL SYSTEM
During embryonic and postnatal development, monoamines modulate neurodevelopmental processes such as cell division, migration and differentiation, axonal and dendritic elaboration and connectivity, and myelination and apoptosis (Gaspar et al, 2003; Haydon et al, 1984, 1987; Lauder, 1990; McCarthy et al, 2007; Popolo et al, 2004; Tarazi et al, 1998b; Teicher et al, 1995). 5-HT, eg, exerts prominent autoregulatory control in dorsal and median raphe nuclei formation during embryonic development by acting on 5-HT1A and 5-HT1B autoreceptors to limit the number of 5-HTergic neurons (Rumajogee et al, 2004). An example for heteroregulation of fundamental developmental processes is its modulatory effect on axonal guidance factors. Through 5-HT1B/1D heteroreceptors, 5-HT reverses the attraction exerted by netrin-1 on the developing posterior dorsal thalamic axons into repulsion, thereby contributing to patterning of thalamocortical connections in the developing brain (Bonnin et al, 2007). The two most prominent examples for 5-HTergic modulation of cortical and subcortical brain organization on a system-wide level relate to somatosensory and visual system formation.
5-HT and Somatosensory System Development
The rodent somatosensory cortex (SSC) contains barrel fields, with individual barrels representing single processing units for each vibrissa (reviewed in (Erzurumlu and Gaspar, 2012; Inan and Crair, 2007; Petersen, 2007; van Kleef et al, 2012)). Barrels reside in layer IV and are organized around thalamocortical projections from the ventroposteromedial (VPM) nucleus of the thalamus (Killackey, 1973). Thalamocortical VPM neurons transiently express 5-HTT and vesicular monoamine transporter 2 (VMAT2) from E15 to P10, allowing for 5-HT uptake and vesicular storage (Hansson et al, 1998; Lebrand et al, 1996; Lebrand et al, 1998). The transient expression of 5-HTT and the maintenance of low local 5-HT levels is critical for the formation of the barrel fields, as monoamine oxidase A (MAOA) or 5-HTT-knockout mice fail to develop barrel fields (Cases et al, 1996; Persico et al, 2001). The sensitive period for 5-HTergic modulation of barrel field formation has been mapped to P0–P4 (Vitalis et al, 1998).
The proposed mechanism by which excess 5-HT leads to disruption of barrel field formation involves 5-HT1B receptors, also transiently expressed by thalamocortical afferents during the first postnatal week in rodents (Bennett-Clarke et al, 1993). VPM 5-HT1B receptors are located on axon terminals and inhibit the release of glutamate relative to incoming stimuli (Laurent et al, 2002; Mooney et al, 1994). Yet, such glutamatergic synaptic neurotransmission is necessary for the formation of barrel columns (Li et al, 2013). Genetically removing 5-HT1B receptors from MAOA- and 5-HTT-knockout mice rescues the formation of barrel fields (Salichon et al, 2001), affirming that the excess extracellular 5-HT availability in MAOA- and 5-HTT-knockout mice disrupts barrel field formation through 5-HT1B receptor activation. Intriguingly, birth reduces extracellular 5-HT levels and this reduction is necessary and sufficient for birth to serve as an initiator for barrel formation (Toda et al, 2013).
Too little 5-HT can also pose problems for barrel field formation. Lesioning 5-HT fibers on the day of birth leads to a 20–30% decrease in the size of barrels at P6 and P60 (Bennett-Clarke et al, 1994), and depleting 5-HT in neonatal pups with the toxin p-chloroamphetamine decreases the number of 5-HTergic axons in the barrel field, and delays the segmentation of thalamocortical projections into individual barrels (Blue et al, 1991). VMAT2-knockout mice, which have virtually undetectable levels of 5-HT, DA and norepinephrine (NE) in the brain, also show a lack of barrel formation in SSC; however, thalamocortical axons segregate properly, with a 1-day delay. This dissociation between the effects of VMAT2 deficiency on layer IV barrels and thalamocortical axons indicates that monoamines are essential for the formation of barrels in the cortex, but not for thalamocortical axon patterning (Alvarez et al, 2002).
5-HT and Visual System Development
In rodents, crossed and uncrossed retinal fibers have a distinct pattern of distribution within the superior colliculus (SC) and the lateral geniculate nucleus (LGN; Reese and Cowey, 1983). This adult pattern is due to spontaneous activity within retinal cells, as well as competition between inputs from the two eyes. Blocking neural activity in one eye prevents normal pattern formation in the SC (Thompson and Holt, 1989), and blocking activity in the target region disrupts pattern development within the LGN (Shatz and Stryker, 1988). In mammals, retinal axons initially innervate the contralateral and ipsilateral LGN in an intermingled fashion. Over a critical period spanning from approximately P3 to P8, ipsilateral and contralateral retinal axons become organized into a predictable layer-dependent pattern of distribution through gradual pruning of inappropriate projections and expansion of correct projections in retinothalamic axons (Sretavan and Shatz, 1986).
Increased 5-HT disrupts normal pattern development in the SC of the Syrian hamster (Mooney et al, 1998), and MAOA-knockout mice show a failure of crossed and uncrossed retinal fibers to segregate in the LGN and the SC (Upton et al, 1999). This disrupted pattern can be rescued by inhibiting 5-HT synthesis during the first 2 weeks of life (Upton et al, 1999). During this 2-week period, retinal fibers transiently express 5-HTT, VMAT2, and HTR1B (Hansson et al, 1998; Lebrand et al, 2006; Upton et al, 2002; Upton et al, 1999). Therefore, a similar mechanism is proposed for the 5-HT-mediated alteration in pattern development in somatosensory and visual systems. Transient 5-HTT expression keeps extracellular 5-HT below a critical concentration. An excess of extracellular 5-HT such as seen in 5-HTT- or MAOA-knockout mice activates 5-HT1B receptors on thalamocortical or retinogeniculate axon terminals, thereby decreasing their excitatory glutamatergic output (Rhoades et al, 1994) and disrupting the activity-dependent maturation of axon collaterals necessary for the segregation of SC and LGN inputs. Interestingly, a lack of 5-HT or 5-HT1B receptors affects the refinement of the SC retinal projection, whereas the establishment of eye-specific patterns in the dorsal LGN appears not to be sensitive (Upton et al, 2002). But as observed for barrel field formation, birth-induced reduction of extracellular 5-HT levels regulates the segregation of retinal ganglion cell axons to the LGN (Toda et al, 2013).
Functional Consequences
5-HTT−/− mice display reduced cerebral glucose utilization in response to whisker stimulation across all levels of somatosensory whisker processing (Esaki et al, 2005), a deficit that is rescued by the administration of the 5-HT synthesis inhibitor parachlorophenylalanine (PCPA). Behavioral deficits that could result from somatosensory or visual system dysfunction include prolonged righting and trembling during locomotion seen in MAOA-deficient pups (Cases et al, 1995) and motor deficits seen in both MAOA- and 5-HTT-deficient adult mice (Bortolato et al, 2013; Cases et al, 1995; Morelli et al, 2011); however, proof of a causal relationship is thus far lacking.
Clinical Relevance
High levels of 5-HT during a sensitive perinatal period cause permanent anatomical defects in the somatosensory and visual system. The underlying mechanisms likely apply to other systems as well, because transient expression of 5-HTT also occurs in other sensory, thalamic, hippocampal, hypothalamic, and prefrontal cortical neurons throughout development in rodents (Lebrand et al, 1996; Lebrand et al, 1998; Narboux-Neme et al, 2008). Transient ectopic 5-HTT expression during development is also observed in non-human primates, eg, in sensory neurons of the common marmoset (Lebrand et al, 2006). In human 12 to 14-week-old fetuses, 5-HTT-immunolabeled fibers have been identified in the rostral and caudal limbs of the internal capsule, including putative thalamocortical fibers that project from the mediodorsal thalamus to the frontal cortex (Verney et al, 2002). Thus, humans and rodents may share a similar role for 5-HT and 5-HT uptake during cortical development. The hypothesized conservation of ectopic 5-HTT expression in humans is of interest because it might relate not only to sensory phenotypes but also to clinical psychopathology.
Many psychiatric and neurodevelopmental disorders, including autism spectrum disorder, attention deficit hyperactivity disorder, developmental coordination disorder, and schizophrenia encompass sensory and/or motor deficits (Butler et al, 2001; Crane et al, 2009; Dewey et al, 2007; Doniger et al, 2002; Piek and Dyck, 2004; Rogers and Ozonoff, 2005). Symptoms vary broadly, manifesting as sensory hypo- or hyper-responsiveness, or problems with sensory filtering. The presence of sensory symptoms in these disorders that are primarily characterized by emotional and cognitive dysfunction supports the hypothesis of common mechanisms underlying sensory/motor and emotional/cognitive phenotypes.
A 5-HT-SENSITIVE DEVELOPMENTAL PERIOD IMPACTING ADULT EMOTIONAL BEHAVIOR AND COGNITION
Though limbic circuits retain some plasticity in adult life, their formation and interconnectivity is predominantly set during embryonic and postnatal development. Heightened plasticity during circuit development bestows malleable potential to environmental and genetic factors (Hensch, 2004; Knudsen, 2004). Hence, much like the maturation of sensory systems, limbic circuit formation may also pass through sensitive developmental periods, during which external and internal factors can impact and modulate circuit formation and consequently, behaviors encoded by them. Such a model is congruent with mood disorders often having their origins in early life (Baram et al, 2012; Caspi et al, 2003; Kendler et al, 1992; Moffitt et al, 2007; Pietrek et al, 2013; Quinn et al, 2013; Wals and Verhulst, 2005). Although our understanding of the molecular factors that define maturing limbic circuit properties is still limited, 5-HT has emerged as one such factor modulating not only adult limbic function, but also limbic circuit formation.
5-HT and Emotional and Cognitive Behavior
5-HT signaling modulates emotional behavior (Arango et al, 2003; Charney, 1998; Fernandez and Gaspar, 2012). Reduced plasma and platelet 5-HT levels as well as blunted prefrontal cortical responses to elevated 5-HT are observed in psychopathological states such as depression, panic disorders, and post-traumatic stress disorder (Anderson et al, 2004; Cannon et al, 2013; Davis et al, 1997; Kovacic et al, 2008; Lesch et al, 1996; Maron et al, 2004; Spivak et al, 1999). Manipulations evoking hypo-5-HTergic states such as tryptophan depletion exert prodepressive effects (Blokland et al, 2002; Feder et al, 2011), whereas selective 5-HT reuptake inhibitors (SSRIs) that block the 5-HTT and hence enhance synaptic 5-HT levels have antidepressant and anxiolytic efficacy (Fuller and Wong, 1990). Preclinical research mirrors this relationship, as reduced firing of 5-HTergic neurons is often observed in animal models of depression and anxiety (Bambico et al, 2009; Challis et al, 2013). Although genome-wide association studies (GWAS) have thus far not identified genetic risk loci for depression (Flint and Kendler, 2014), the relationship between perturbed 5-HTergic signaling and affective behavior is further supported by genetic linkage and association studies. Congruent with the generally positive correlation of 5-HTergic phenotypes and emotion, low-expressing alleles of TPH2 coding for the 5-HT-synthesizing enzyme tryptophan hydroxylase 2 (Gutknecht et al, 2007; Reuter et al, 2007; Zill et al, 2004) and the high-expressing variant of the presynaptic inhibitory receptor HTR1A (Lemonde et al, 2003; Schmitz et al, 2009) are associated with negative emotionality and enhanced predisposition to depression and suicidality. However, polymorphisms in the regulatory regions of MAOA, which negatively influence its transcriptional levels (Sabol et al, 1998), are also associated with enhanced anxiety and depressive behavior (Schmidt et al, 2000; Tadic et al, 2003). Likewise, the hypomorphic short (s) allelic variant of the 5-HTT gene-linked polymorphic region (5-HTTLPR) is associated with trait anxiety and ‘neuroticism’, and increased susceptibility to environmental stress in some (Grabe et al, 2012; Lesch et al, 1996; Xie et al, 2009), albeit not all studies (Fisher et al, 2012; Peyrot et al, 2013). These surprising associations are in line with reduced expression of 5-HTT found in brains of depressed individuals and suicide victims (Underwood et al, 2012; Willeit et al, 2000). Anxiogenic associations with hypomorphic MAOA and 5-HTT alleles are opposite to what one would predict based on the anxiolytic and antidepressant effects of monoamine oxidase inhibitors and drugs blocking the 5-HTT (Fuller and Wong, 1990). However, pharmacological treatments usually circumvent developmental periods, whereas genetic factors act throughout life, including development. Hence, these results suggest that elevated 5-HT levels may exert starkly contrasting effects on emotional behavior, depending upon the age of exposure.
Both non-human primate and rodent models support a central role for 5-HTergic genotypes in establishing psychiatric vulnerability. MAOA-deficient mice exhibit enhanced startle behavior and decreased exploration of novel environments (Cases et al, 1995; Chen et al, 2004). These behavioral phenotypes correlate with increased monoamine levels specifically in postnatal life, as the gradual rise in monoamine oxidase B (MAOB) normalizes adult monoamine metabolism (Tsang et al, 1986). Whereas MAOA activity impacts 5-HT, DA, and NE levels, 5-HTT function selectively regulates 5-HT signaling. In 5-HTT−/− mice, elevated extracellular 5-HT correlates with enhanced anxiety, learned helplessness, behavioral despair, and impaired social interaction (Ansorge et al, 2004; Kalueff et al, 2007; Lira et al, 2003; Moy et al, 2009; Muller et al, 2011). 5-HTT-knockout animals also exhibit impaired extinction recall of fearful memories (Wellman et al, 2007). Loss-of-function and hypomorphic 5-HTT variants also enhance the susceptibility to adverse behavioral effects of stressors. Rhesus macaques carrying an orthologue of the 5-HTT s allele and 5-HTT−/− mice exhibit exaggerated behavioral and neuroendocrine responses to mild stress, particularly when also exposed to early-life stress (Adamec et al, 2006; Jiang et al, 2009). Resembling the phenotype of 5-HTT-deficient mice, loss of all or only presynaptic inhibitory 5-HT1A receptors results in increased anxiety-related behaviors (Gross et al, 2002; Heisler et al, 1998; Parks et al, 1998; Ramboz et al, 1998; Richardson-Jones et al, 2011).
Indicating a linear relationship between developmental 5-HT tone and adult emotional behavior, multiple transgenic models of life-long hypo-5-HTergic function, including those defective in raphe specification (LMX1b-PET1Cre, PET1-Tox, PET1−/−), 5-HT packaging (VMAT2-SERTCre), or exhibiting enhanced reuptake of synaptic 5-HT (5-HTT-overexpressing mice), display enhanced exploration of novel environments and reduced innate anxiety (Dai et al, 2008; Jennings et al, 2006; Kiyasova et al, 2011a; Narboux-Neme et al, 2011). However, some constitutive mutants for genes involved in 5-HTergic fate specification (PET1−/−) and synthesis (TPH2−/−) with drastically reduced 5-HT levels exhibit prodepressive and anxiogenic behavior (Beaulieu et al, 2008; Hendricks et al, 2003). Hence, a simple linear relationship breaks apart at extreme ends, suggesting that both severe depletion and elevation of 5-HT during development adversely influences adult emotional behaviors, but still supporting the core hypothesis that developmental 5-HT signaling establishes the set point for adult emotionality.
Direct proof of this hypothesis requires temporal control over 5-HTergic parameters, which constitutive genetic models lack. Pharmacologic probing of the 5-HTsystem during development lacks 100% target specificity but has nevertheless proven invaluable in advancing our knowledge of 5-HT-sensitive periods (Table 1). For example, animals administered SSRIs or 5-HTT blocking tricyclic antidepressants during the first 3 weeks of postnatal life (P4–P21), but not in adulthood (P90–P107 or P56–P70), mimic the prodepressive and anxiogenic phenotype observed in transgenic models with constitutively enhanced 5-HTergic tone (Ansorge et al, 2004, 2008; Popa et al, 2008). We have recently refined this SSRI-sensitive period to P2–P11, and extended the behavioral characterization, finding impaired hippocampal-dependent spatial learning and contextual fear learning, as well as diminished amygdala and PFC-dependent fear extinction and extinction recall (Rebello et al, 2014). This P2–P11 period, interestingly, not only lies within the maturation period of both 5-HTergic afferents and cortical circuits (Kiyasova and Gaspar, 2011b; Lidov and Molliver, 1982a; Lidov and Molliver, 1982a; Vitalis et al, 2013), but also coincides with the peak of cortical 5-HT and 5-HT metabolite levels (Hohmann et al, 1988; Figure 1). Such tight confinement of this sensitive period, however, does not persist when comparing between species. For example, although in mice, PA SSRI exposure lacks persistent consequences on fear-, anxiety-, or stress-related behaviors (Norcross et al, 2008; Yu et al, 2014), rats exhibit increases in anxiety-like behavior following P25–P46, P35–P49, or P67–P88 5-HTT blockade (Iniguez et al, 2010, 2014; Karpova et al, 2009). Likewise, although in mice, 5-HTT blockade between P2 and P11 impairs cognitive behavior (Rebello et al, 2014), pharmacological perturbations that enhance levels of 5-HT from P11 to P20 but not from P1 to P10 result in dose-related impairments of sequential learning and spatial learning and memory in rats (Broening et al, 2001; Morford et al, 2002). These findings suggest that 5-HT-sensitive periods exhibit species-specific characteristics. Furthermore, specific behavioral phenotypes resulting from postnatal SSRI treatment appear to exhibit differential sensitivity to the timing of SSRI treatment. Interestingly, both rat and mouse studies have revealed timing-dependent bidirectional effects of chronic 5-HTT blockade. Although clomipramine or SSRI exposure in rats from P8 to P21 causes enhanced immobility in the forced swim test (Hansen et al, 1997; Vogel et al, 1990); PA (P35–P49), but not adult (P65–P79), fluoxetine (FLX) exposure reduces floating (Iniguez et al, 2010). In mice, P2–P11 FLX exposure increases forced swim test immobility (Rebello et al, 2014), whereas P4–P21 or P35–P49 FLX exposure reduces floating (Iniguez et al, 2014; Karpova et al, 2009).
Importantly, developmentally blocking the other two major brakes of 5-HT signaling, MAOA and 5-HT1A autoreceptors, results in comparable behavioral sequelae, but again, some differences exist with regard to timing (Table 1). The refined 5-HTT blockade sensitive period (P2–P11) overlaps with the MAOA blockade sensitive period (P2–P21; Yu et al, 2014), but dissociates from the 5-HT1A receptor blockade sensitive period (Donaldson et al, 2014; Gross et al, 2002; Lo Iacono and Gross, 2008; Richardson-Jones et al, 2011; Vinkers et al, 2010). Pharmacological 5-HT1A receptor blockade from P0 to P21 (Gross et al, 2002; Vinkers et al, 2010) or P13 to P34 (Lo Iacono and Gross, 2008), is sufficient to elicit the adult anxiety phenotype. Furthermore, the suppression of endogenous HTR1A autoreceptor expression throughout life or from P2 to P30 is sufficient to increase anxiety in the adult (Donaldson et al, 2014; Richardson-Jones et al, 2011).
Overall, even with the species and target effects taken into account, the convergence of behavioral malleability when interfering with 5-HT signaling during early postnatal periods highlights the importance of this time window for circuit maturation and circuit plasticity.
Mechanistic Insight on the Postnatal Establishment of Perturbed Emotionality
Elevated postnatal 5-HT levels may exert their effects by impinging on the normal developmental trajectory of both its target limbic neurocircuits as well as the 5-HTergic system itself. For example, the autoinhibitory effect of 5-HT on 5-HTergic differentiation reduces 5-HT neuronal numbers in embryonic raphe cultures (Rumajogee et al, 2004) and 5-HTT−/− mice (Lira et al, 2003). Though these changes might carry their own behavioral consequences, they likely dissociate from the effects produced by increased early postnatal 5-HT signaling, because 5-HTergic cell numbers are set at that time point. However, additional aspects of 5-HTergic function are still malleable during postnatal periods (Figure 2). 5-HTergic neurons of the dorsal raphe fire at a fourfold lower rate in 5-HTT−/− mice when compared with controls (Lira et al, 2003). Likewise, rats exposed to clomipramine from P8 to P21 and mice exposed to FLX from P4 to P21 also have reduced 5-HTergic neuronal activity when compared with vehicle-treated controls in adulthood (Kinney et al, 1997; personal communication MSA). Furthermore, 5-HTergic fiber density in the HPC and medial PFC (mPFC) of rats treated with citalopram (Maciag et al, 2006; Weaver et al, 2010) or mice treated with FLX (personal communication MSA) during postnatal development is reduced. These anatomical abnormalities might synergize with hypo-5-HTergic tone to weaken functional connectivity and thus 5-HTergic modulation of the HPC and mPFC, which in turn could underlie increased anxiety/depression and impaired cognition. Testing of such direct causal relationships between circuit-specific 5-HTergic input and behavior are underway in many labs that are applying optogenetic and pharmacogenetic tools to decipher the 5-HTergic code in vivo. For example, a recent study has uncovered a role for 5-HT in encoding reward, wherein the enhanced activity of dorsal raphe neurons is observed in reward-associated tasks, and optogenetic manipulation of 5-HTergic neuronal activity strongly biases reward-associated behaviors (Liu et al, 2014).
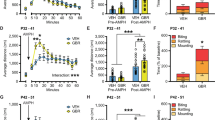
The 5-HT-sensitive developmental period—neural correlates of behavioral sequelae. (a) Diagram of critical developmental processes that overlap temporally with early postnatal SSRI treatment (P2–P11). Due to the temporal overlap, these processes provide candidate mechanistic underpinnings of early postnatal SSRI-induced alterations in affective behaviors. (b) Key nodes involved in anxiety and depressive behaviors and their basic functions and connectivity in the normal adult brain. (c) Summary of alterations occurring within the key nodes of the circuit as a result of developmental 5-HT elevation, and the resulting anxiety and depressive behaviors seen during adulthood. AMY, amygdala; mPFC, medial prefrontal cortex; HPC, hippocampus; DR, dorsal raphe nucleus; MR, median raphe nucleus; IL, infralimbic cortex; SSRI, selective 5-HT reuptake inhibitor; 5-HT, serotonin.
In addition to autoregulation, 5-HT acts via 14+ heteroreceptors located on a vast and diverse population of postsynaptic target cells. With these heteroreceptors emerging early in development, it is likely that enhanced levels of early postnatal 5-HT act via heteroreceptors to influence postsynaptic circuit maturation thus evoking structural, physiological, and behavioral consequences. Several specific 5-HT heteroreceptors have emerged as potential candidates in mediating 5-HT-sensitive period consequences. For example, 5-HT2A receptors in the forebrain have a key role in the regulation of anxiety behavior, as their constitutive ablation reduces conflict anxiety (Weisstaub et al, 2006). As adult treatment with 5-HT2A antagonists fails to evoke similar anxiolytic effects (Griebel et al, 1997; Kehne et al, 1996), the HTR2A−/− phenotype might have developmental origins. Conversely, increased 5-HT2A receptor signaling during development would be predicted to exert anxiogenic consequences in adulthood. Indeed, postnatal blockade of the 5-HT2A and 5-HT2C receptors prevents the emergence of early SSRI-evoked anxiety and depressive behavior (Sarkar et al, 2013). The dynamic developmental expression characteristics of cortical HTR2A further support a causal role for this particular receptor in mediating the effects of early postnatal 5-HTT blockade: the shift of cortical neuron responses to 5-HT, from 5-HT2A-mediated excitation in early postnatal life to predominantly inhibitory responses past P15 could explain the extent of the murine 5-HTT sensitive period (Beique et al, 2004; Zhang, 2003).
5-HT3A receptors have been invoked as critical mediators of developmental 5-HT signaling through studies of cortical cytoarchitecture. Postnatal FLX treatment reduces the arborization of apical dendrites of layer 2/3 infralimbic (IL) but not prelimbic (PL) pyramidal neurons in mice (Rebello et al, 2014; Figure 2). A similar 5-HT sensitivity has been reported for layer 2/3 pyramidal neurons in the SSC, where increased 5-HT signaling from E8 to E18 decreases apical dendritic arborization (Chameau et al, 2009; Smit-Rigter et al, 2012). This latter effect is mediated by 5-HT3A receptors present on reelin secreting Cajal–Retzius cells (Smit-Rigter et al, 2012), which upon activation stimulate the release of reelin, which in turn limits cortical neuron apical dendritic elaboration (Chameau et al, 2009; Smit-Rigter et al, 2011). As Cajal–Retzius cells are still present and active in the first two postnatal weeks, a similar mechanism involving hyperactivation of the 5-HT3Areceptors can be postulated for the cytoarchitectural changes observed following postnatal FLX treatment. Differential activity or sensitivity of this pathway as a function of time and region might underlie the restriction of postnatal SSRI consequences to the apical arbors of IL and not PL neurons. An interesting, yet unstudied, aspect in that regard is whether developmental 5-HT signaling permanently impacts the cytoarchitecture and consequently function of Cajal–Retzius cells themselves and/or other HTR3A expressing interneurons such as neurogliaform cells.
5-HT1B receptors are also strong candidates for mediating the effects of excessive early-life 5-HT on cytoarchitecture. The critical role of 5-HT1B receptors in shaping sensory system development can be hypothetically transposed to the maturation of non-sensory thalamocortical connectivity and cortical architecture, as well as hippocampal wiring. Neurons of the thalamic medial dorsal nucleus that project to the mPFC and influence pyramidal cell dendritic arborization (Marmolejo et al, 2012), express high levels of HTR1B (Bonnin et al, 2006), 5-HTT, and VMAT2 in early postnatal life (Lebrand et al, 1998). Therefore, 5-HT could act through 5-HT1B receptors to modulate the activity of thalamocortical afferents projecting to the PFC and thus influence thalamocortical axonal fields and cortical cytoarchitecture, thereby contributing to the postnatal SSRI-induced structural changes in the PFC.
Taken together, current preclinical data allow us to conclude that increased extracellular 5-HT during P2–P11, regardless of the primary cause, elicits adult anxiety and depression-like behavior and impairs cognition in rodents. Mechanistic studies highlight that 5-HT receptor diversity results in pleiotropic effects of 5-HT during development to elicit these long-lasting changes in emotional and cognitive behavior (Figure 2). Most mechanistic insight has related specific receptors to specific neuroanatomical and neurophysiological phenotypes. How such phenotypes relate to adult behavior remains to be established for most cases. Optogenetic and pharmacogenetic techniques have proven invaluable for testing causal relationships between neurophysiology and behavior. Tools to manipulate and sculpt cytoarchitecture and circuit connectivity remain to be established.
Early-Life Adversity and 5-HT Perturbation
Exposure to diverse early-life stressors including inadequate maternal care, maternal separation, and novelty exposure evoke persistent enhancements in anxiety and depression-related behaviors, altered cognitive function, and dysregulated neuroendocrine responses to adult stressors (Kalinichev et al, 2002; Lehmann et al, 1999; Suri et al, 2013). Concomitantly, early-life adverse experience evokes 5-HTergic dysregulation in mice. For example, early-life stress exposure increases postnatal 5-HT and 5-HT metabolite levels in the dorsal raphe and its limbic projection areas, the amygdala, the HPC, and the mPFC (Franklin et al, 2011; Ohta et al, 2014; Raftogianni et al, 2012; Rentesi et al, 2013), and reduces dorsal raphe HTR1A expression and 5-HT1A receptor levels (Leventopoulos et al, 2009; Ohta et al, 2014), together strongly indicative of a postnatal hyper-5-HTergic phenotype. Furthermore, maternal separation increases prefrontal 5-HT2A/2C receptor function during the second postnatal week (Benekareddy et al, 2010) and blockade of 5-HT2A/2C function during maternal separation prevents the emergence of the adverse early stress-evoked behavioral and molecular sequelae (Benekareddy et al, 2011). Together, these findings strongly indicate that early-life stress acts through early postnatal 5-HT-sensitive period interference to impact adult behavior.
If this model is broadly applicable, then early-life stress should interact with genetic factors that also alter 5-HT signaling to confer risk for later life emotional dysfunction. Indeed, some studies indicate that 5-HTT genotypes interact with life history to determine adult vulnerability to psychopathology: eg, individuals carrying the s allele are more likely to suffer from depression only in the background of stressful life history, and in particular when adverse early events were experienced (Caspi et al, 2003; Grabe et al, 2012; Xie et al, 2009). All positive candidate gene linkage and association studies for major depression and related disorders have come under criticism, because GWAS have so far failed to independently identify risk alleles (Flint and Kendler, 2014). However, because the study of human environmental factors, as well as insight gained through the work on non-human primate and rodent models, converge to highlight the importance of developmental 5-HT, we predict that GWAS will eventually detect genetic risk factors which likewise impinge on developmental 5-HT signaling.
Clinical Relevance
Basic research has strongly shaped the model in which maturing 5-HTergic input during developmental periods has an instructive role in the maturation of limbic neurocircuits, and dysregulation of 5-HTergic signaling impinges on normal development to evoke persistent changes in emotionality. Early human studies investigating this relationship have focused greatly on the 5-HTTLPR variants, and their role in psychopathology, with many but not all studies reporting an association between the low-expressing s allele and increased trait anxiety (Lesch et al, 1996; Schinka et al, 2004; Sen et al, 2004), increased incidence and severity of major depressive disorder following a stressful life event (Caspi et al, 2003; Culverhouse et al, 2013; Karg et al, 2011; Munafo et al, 2009; Risch et al, 2009; Zalsman et al, 2006), and heightened behavioral reactivity to fearful stimuli (Armbruster et al, 2009; Brocke et al, 2006). A common 5-HTT-polyadenylation polymorphism reducing 5-HTT expression is associated with fear extinction recall deficits (Hartley et al, 2012; Yoon et al, 2013).
Consistent with these behavioral data, brain imaging studies demonstrate an association between the s allele and higher levels of amygdala activation in response to fearful stimuli (Furmark et al, 2004; Hariri et al, 2002; Kobiella et al, 2011; Murphy et al, 2013; Scharinger et al, 2010), reduced grey matter volumes in the dorsolateral PFC, amygdala and the HPC (Atmaca et al, 2011; Frodl et al, 2008; Kobiella et al, 2011; Pezawas et al, 2005), microstructural changes in the uncinate fasciclus, a white matter tract connecting limbic and frontal areas, including the amygdala and anterior cingulate cortex (Pacheco et al, 2009), and decreased coupling of the amygdala-anterior cingulate circuit (Heinz et al, 2005; Lemogne et al, 2011; Pezawas et al, 2005; Roiser et al, 2009; Shah et al, 2009; Volman et al, 2013). Importantly, functional coupling between the anterior cingulate cortex and the amygdala is correlated with trait anxiety (Hahn et al, 2011; Prater et al, 2013).
Although the influence of the 5-HTTLPR on promoter activity and the production of mRNA, protein product, and 5-HT reuptake activity has been documented in cellular assays (Bradley et al, 2005; Heils et al, 1996; Lesch et al, 1996; Philibert et al, 2008; Stoltenberg et al, 2002), paradoxically nearly all attempts to examine the influence of 5-HTTLPR promoter variants on 5-HTT levels in adult brain have been negative (Lim et al, 2006; Murthy et al, 2010; Oquendo et al, 2007; Parsey et al, 2006; Preuss et al, 2000). This disconnect leaves open the possibility of a more prominent impact of the 5-HTTLPR on developmental 5-HTT expression, including transient 5-HTT expression during human fetal development. Such a role would be congruent with clinical and endophenotype-related associations originating during sensitive developmental periods.
The 5-HT-sensitive murine P2–P11 period roughly corresponds to the 3rd trimester of human gestation. Consequently, preclinical findings become relevant in the context of SSRI use during pregnancy. Up to 8% of pregnant women are reported to use SSRIs, and their use during pregnancy is currently increasing (Andrade et al, 2008; Bakker et al, 2008). SSRIs cross the placental barrier (Hendrick et al, 2003; Rampono et al, 2004) and can be measured in amniotic fluid (Loughhead et al, 2006). Although SSRIs do not cause any overt developmental abnormalities in the neonates (Chambers et al, 1996; Pastuszak et al, 1993; Simon et al, 2002), in utero exposure to SSRIs increases the risk of preterm birth and lower birth weights (El Marroun et al, 2012; Grzeskowiak et al, 2012; Oberlander et al, 2006). Furthermore, an association between the use of SSRIs during pregnancy and persistent pulmonary hypertension (Chambers et al, 2006; Kieler et al, 2012), congenital cardiac defects (Berard et al, 2007; Diav-Citrin et al, 2008; Pedersen et al, 2009) and a slight delay in motor development (Casper et al, 2003; de Vries et al, 2013; Hanley et al, 2013) have been noted in the neonates. Exposed infants also display indications of central nervous system stress at 3 weeks after birth (Salisbury et al, 2011), and affected neurological functioning as measured by general movement at 3–4 months postpartum (de Vries et al, 2013). Symptoms appear to be moderated by infant 5-HTTLPR, as infants homozygous for the s allele display higher severity of symptoms (Oberlander et al, 2008), reinforcing the hypothesis that a high level of 5-HT during development is detrimental. To date, little is known about the long-term impact of in utero SSRI exposure on brain development, adult behavior, and the prevalence of emotional disorders later in life. Recent studies have suggested enhanced internalizing behavior in childhood (Oberlander et al, 2010), and an increased risk of autism spectrum disorders in prenatally SSRI-exposed offspring (Croen et al, 2011; Rai et al, 2013). These findings are in line with an association noted between the 5-HTTLPR and specific deficits observed in autism spectrum disorders, whereby those possessing the s allele demonstrate higher deficiencies in nonverbal and social behaviors (Brune et al, 2006). Still, further research is needed to determine whether associations with SSRI exposure are causal, and to disentangle the effects of maternal depression versus maternal SSRI use on infant and childhood emotional behavior (Misri et al, 2006; Oberlander et al, 2010; Oberlander et al, 2007; Salisbury et al, 2011). Although small, non-population-based cohort studies observed no excess risk by age 3 to 7 (Nulman et al, 1997; Nulman et al, 2002), more longitudinal studies are required to assess the long-term effects of postnatal SSRI exposure on the development of psychiatric disorders (Malm et al, 2012). Ultimately, we hope that within the next 5 years, such longitudinal as well as additional epidemiological population-based studies will produce data that will allow clinicians to better weigh risks and benefits when confronted with depression during pregnancy.
A 5-HT- AND DA-SENSITIVE PERIOD IMPACTING ADULT AGGRESSION
Aggression is a behavioral construct often subdivided along defining characteristics, such as target, mode, or cause of aggression. The most frequent distinction occurs between premeditated violence, which represents a planned behavior and is associated with low autonomic response, and impulsive aggression, which is reactive and associated with high autonomic response (Barratt and Felthous, 2003; Gollan et al, 2005; Meloy, 2006). Adult aggression is critically regulated by monoamine signaling, 5-HT, and DA signaling in particular. These two monoamines appear to have generally opposing roles, with DA promoting and 5-HT inhibiting aggression.
DA and Aggressive Behavior
Hyperactivity of the DA system is associated with increased impulsive aggression. In animals, nucleus accumbens (NAc) DA release increases in anticipation of aggressive episodes (Ferrari et al, 2003; Malison et al, 1998), and NAc and PFC release increases during and following aggressive encounters in rats (Tidey and Miczek, 1996; van Erp and Miczek, 2000). Systemic administration of methamphetamine or the DA receptor agonist apomorphine decreases the threshold for defensive attack behavior elicited by electrical stimulation of the ventromedial hypothalamic (VMH) nucleus in cats (Maeda and Maki, 1986; Maeda et al, 1985). Conversely, systemic administration of the D1/D2 receptor antagonist risperidone (Rodriguez-Arias et al, 1998), the D2 receptor antagonist raclopride (Aguilar et al, 1994), and the D1 antagonist SCH23390 (Rodriguez-Arias et al, 1998) all reduce aggression. Furthermore, blockade of D1 or D2 receptors in the NAc attenuates aggression in mice (Couppis and Kennedy, 2008). Mice lacking DAT exhibit a hyper-DAergic tone, which correlates with hyper locomotion (Giros et al, 1996), and increased reactive aggression following mild social contact (Rodriguiz et al, 2004). Moreover, cocaine, which blocks the DAT, significantly escalates aggression when administered during adolescence (P27–P57; DeLeon et al, 2002; Harrison et al, 2000), and methamphetamine significantly increases aggression in male mice when administered chronically (Sokolov and Cadet, 2006; Sokolov et al, 2004). Heterozygous catechol-o-methyl transferase (COMT)-deficient male mice exhibit increased frontal cortex DA levels and increased aggression (Gogos et al, 1998). Lastly, specific activation of the VTA DAergic neuronal activity using optogenetic stimulation increases isolation-induced aggression (Yu et al, 2014).
Results from preclinical animal models are congruent with human studies. For example, levels of DA metabolites in the cerebrospinal fluid (CSF) of violent offenders positively correlate with psychopathy (Soderstrom et al, 2001). Typical and atypical antipsychotic agents that antagonize the D2 receptor attenuate pathological aggression (Brizer, 1988; Chengappa et al, 1999; Dorevitch et al, 1999; Lenox et al, 1992; Lerner et al, 1979; Rocca et al, 2002; Schulz et al, 1999). However, low levels of D2/D3 receptors in the rodent NAc and decreased D2/D3 receptor binding in the midbrain of the human, are likewise correlated with impulsive behavior (Buckholtz et al, 2010b; Dalley et al, 2007), still highlighting the DA system but possibly suggesting compensatory adjustment. Human genetic-association studies have also linked the DA system to aggression. Among people with a diagnosis of personality disorder, the low-expressing G allele of the COMT rs165599 SNP is associated with self-reported aggression (Flory et al, 2007). Likewise, among individuals diagnosed with schizophrenia, the low-activity Met allele of the COMT Val158Met polymorphism is associated with high aggression (Bhakta et al, 2012; Gu et al, 2009; Han et al, 2004; Hong et al, 2008; Koh et al, 2012; Kotler et al, 1999; Lachman et al, 1998; Singh et al, 2012; Strous et al, 2003; Volavka et al, 2004c). DRD2 and DRD4 gene variants interact to predict adolescent conduct disorder and adult antisocial behavior (Beaver et al, 2007), as well as dysfunctional impulsivity (Colzato et al, 2010), and differences in inhibitory control are associated with the DRD4 VNTR polymorphism (Congdon et al, 2008). Collectively, these studies support the DA hypothesis of aggression, which states that DAergic hyperfunction increases aggression (de Almeida et al, 2005a; Seo et al, 2008).
5-HT and Aggressive Behavior
Hypoactivity of the 5-HT system is also correlated with increased impulsive aggression. In rats, prefrontal extracellular 5-HT declines to 80% of baseline levels during aggressive encounters (van Erp and Miczek, 2000). In rhesus macaques and vervet monkeys, levels of the 5-HT metabolite 5-hydroxyindoleacetic acid (5-HIAA) in the CSF are negatively correlated with aggression (Higley et al, 1992, 1996a, 1996c), risk taking (Higley et al, 1996b), and impulsivity (Fairbanks et al, 2001; Mehlman et al, 1994). Furthermore, manipulations that lower 5-HTergic signaling, such as PCPA injections, increase impulsivity and aggression, whereas increasing 5-HT signaling using 5-HT precursors or SSRIs can reduce aggressive behavior in rodents (Chiavegatto et al, 2001; Di Chiara et al, 1971; Hodge and Butcher, 1974; Koe and Weissman, 1966; Miczek et al, 2001). Pointing at a critical role for 5-HT1 receptor subtypes in mediating the antiaggressive effects of increased 5-HT signaling, systemic administration of drugs activating 5-HT1A and 5-HT1B receptors exert antiaggressive effects (Bannai et al, 2007; Centenaro et al, 2008; de Boer and Koolhaas, 2005; Miczek et al, 1989; Olivier et al, 1995; Sijbesma et al, 1991). The link between 5-HT and aggression has been further established using genetically modified mouse models. Pet1 knockout mice, which have an 80% reduction in the number of 5-HTergic neurons, exhibit increased aggression (Hendricks et al, 2003). Likewise, life-long 5-HT depletion resulting from TPH2 deletion (Alenina et al, 2009; Angoa-Perez et al, 2012; Mosienko et al, 2012) or Tph2 hypofunction (Beaulieu et al, 2008) increases adult aggression and impulsivity. Conversely, in mice lacking the 5-HTT, increased extracellular 5-HT is associated with reduced aggression and social approach behavior (Bengel et al, 1998; Holmes et al, 2002; Kim et al, 2005; Mathews et al, 2004; Page et al, 2009). Supporting the model in which 5-HT1B receptor signaling exerts inhibitory control over aggressive behavior, male mice that lack 5-HT1B receptors exhibit increased aggressive behavior (Brunner and Hen, 1997; Saudou et al, 1994; Zhuang et al, 1999). Finally, decreasing 5-HTergic activity during adulthood using a pharmacogenetic approach increases territorial isolation-induced aggression using the resident–intruder assay (Audero et al, 2013).
In humans, 5-HTergic hypofunction and impulsive aggression are also often associated. Neurochemical studies find low concentrations of CSF 5-HIAA associated with impulsivity and aggression in many cohorts (Brown et al, 1979, 1982; Kruesi et al, 1990, 1992; Linnoila et al, 1983; Virkkunen et al, 1995; Virkkunen et al, 1994). Several studies also report a blunted neuroendocrine and central metabolic response to a pharmacological 5-HT challenge using fenfluramine in individuals with high aggression (Coccaro et al, 1989, 1996, 1997b; Siever et al, 1999). Importantly, the endocrine response to fenfluramine challenge also inversely correlates with self-rated aggression and impulsivity in a group of healthy controls (Manuck et al, 1998). Conversely, SSRIs reduce impulsive aggression (Berman et al, 2009; Coccaro and Kavoussi, 1997a), and signaling through the 5-HT2A and 5-HT2C receptors exerts opposing effects on impulsive behavior, with 5-HT2A antagonists reducing and 5-HT2C antagonists increasing impulsivity (Krakowski et al, 2006; Winstanley et al, 2004). Furthermore, orbitofrontal 5-HT2A receptor availability is increased in physically aggressive personality disorder patients (Rosell et al, 2010; Soloff et al, 2007).
Human genetic-association studies have also linked the 5-HT system to aggression. Allelic variants in the 5-HTT and TPH1 are associated with aggression in some studies (Davidge et al, 2004; Patkar et al, 2002; Volavka et al, 2004c; Winstanley et al, 2004), and a TPH2 haplotype has been associated with suicidal/parasuicidal behavior and aggression scores (Perez-Rodriguez et al, 2010). Furthermore, the Tyr452 allele of the HTR2A has been associated with childhood onset aggression (Mik et al, 2007). Considered together, studies support the 5-HT hypothesis of aggression, which states that 5-HTergic hypofunction increases aggression.
A Developmental Role for 5-HT and DA in Regulating Aggressive Behavior
Although many principal roles of 5-HT and DA signaling in modulating aggression have been established, many seemingly paradoxical experimental results reveal the divergent consequences of manipulating levels of these neurotransmitters as a function of time. For example, unlike the anxiolytic effects of pharmacologic MAOA inhibition in adulthood, constitutive loss-of-function mutations of MAOA result in a syndrome characterized by antisocial/aggressive behavior in humans (Brunner et al, 1993). Consistently, mice with genetic inactivation of MAOA (MAOA−/−) exhibit not only neophobia but also heightened levels of aggression (Cases et al, 1995; Godar et al, 2010; Scott et al, 2008). The divergent effects of genetic (life-long) mutations versus pharmacologic inhibition (during adulthood) suggest that perturbed monoamine signaling during sensitive periods of brain maturation differentially modulates adult aggression. Several studies now support this hypothesis (Table 1). For instance, adult aggressive behavior is sensitive to PA (P22–P41) DA- and 5-HT manipulations (Yu et al, 2014). Specifically, transient MAOA and DAT blockade during PA mimics the adult hyperaggressive phenotype found in MAOA-deficient mice, whereas transient postnatal (P2–P21) or adult (P180–P201) MAOA blockade does not impact adult aggressive behavior. These temporal characteristics establish the existence of a sensitive period. Interestingly, 5-HTT blockade during that same PA period mimics the adult hypoaggressive phenotype found in 5-HTT-deficient mice, suggesting that a common underlying developmental process is modulated bidirectionally through DA and 5-HT. These findings can explain the increased aggression seen in constitutive MAOA, DAT, and COMT loss-of-function mouse lines (Cases et al, 1995; Gogos et al, 1998; Rodriguiz et al, 2004; Scott et al, 2008), and also support a developmental mechanism for the low-aggression phenotype of 5-HTT−/− mice (Holmes et al, 2002).
PA monoaminergic manipulations also alter the behavioral response to amphetamine challenge in adulthood, with transient MAOA or DAT blockade increasing, and transient 5-HTT blockade reducing locomotor activity after amphetamine challenge (Yu et al, 2014) (Table 1). Because altered amphetamine response is an indication for altered DAergic function, and with the causal link between VTA activity and aggression established, these data indicate that PA monoamine signaling permanently impacts the DAergic system, setting its activity/sensitivity and thereby determining baseline aggression levels. Whether this model of causal relationships applies or whether altered aggression and altered amphetamine response are independent consequences of interfering with adolescent monoamine signaling remains to be established.
Intriguingly, transient pharmacological DAT blockade from P11 to P20 and P20 to P35 in rats has the opposite effect on the behavioral response to stimulant exposure in adulthood, diminishing the locomotor response to cocaine challenge, while transient methylphenidate exposure during adulthood does not alter the motor response to cocaine (Andersen et al, 2002, 2005; Dow-Edwards and Busidan, 2001; Mague et al, 2005) (Table 1). It will be interesting to determine if aggressive behavior is analogously affected. Currently, these findings indicate that the sensitive period for the potentiating effect of the stimulant exposure (and possibly the aggression-increasing effect of stimulant exposure) might reside in a narrower window, which is positioned in late rather than early adolescence. Indeed, rats exposed to cocaine from P28 to P34 exhibit sensitized responses to cocaine challenge at P37, P48, and P96 (Brandon et al, 2001; Table 1). Likewise, preliminary data from our lab indicate that P32–P41 DAT blockade is sufficient to increase adult aggression and amphetamine response in mice (personal communication MSA; Table 1). These findings demonstrate that altered DAergic signaling can impact adult behavior as a function of developmental timing. Together, these data therefore suggest that permanent changes in aggressive behavior and stimulant sensitivity are jointly defined based on the developmental period during which monoaminergic interference occurs, the monoamine system targeted by the interference, and the valence of the interference.
Mechanistic Insight on the PA Establishment of Perturbed Emotionality
The classic aggression circuitry involves hypothalamic areas and the periaqueductal gray (PAG), with upstream control of these regions being provided by the septum, the amygdala, and PFC (Dalley et al, 2011; Davidson et al, 2000; Pavlov et al, 2012) (Figure 3). Electrical stimulation of the intermediate hypothalamic area and the VMH (the ‘hypothalamic attack area’) leads to aggressive behavior in cats and rats (Hess, 1928; Kruk et al, 1979, 1983; Lammers et al, 1988; Roeling et al, 1994; Wasman and Flynn, 1962). More recently, pharmacogenetic and optogenetic studies have confirmed these findings in mice, and have refined neuroanatomical, cellular, and conceptual insight. Specifically, optogenetic stimulation of neurons in the ventrolateral subdivision of the VMH (VMHvl) causes male mice to attack males, females, and inanimate objects, whereas pharmacogenetic silencing of the VMHvl reversibly inhibits aggression, demonstrating both necessity and sufficiency of this region in certain aggressive behaviors (Lin et al, 2011). Intriguingly, the neuronal population responding to aggressive situations is intermingled and overlapping with neurons, which are active during mating (Lin et al, 2011). VMHvl neurons send axons to the dorsolateral PAG, electrical stimulation of which also triggers aggression in cats, suggesting that this hypothalamic-PAG pathway is central to the mediation of aggressive behavior (Siegel and Shaikh, 1997). The VMHvl receives afferents from the PFC, the lateral septum, the bed nucleus of the stria terminalis (BNST), the amygdala, and other hypothalamic regions (Toth et al, 2010), all of which modulate aggression. Most strikingly, lesions to the rostral lateral septum cause hyperdefensiveness/hyperirritability, a phenomenon called ‘septal rage’ (Sodetz and Bunnell, 1970). Conversely, septal stimulation decreases aggression (Potegal et al, 1980). The amygdala receives input from the HPC, and many cortical and thalamic areas, and sends afferents to the hypothalamus and PAG (Gregg and Siegel, 2001).The amygdala thereby integrates sensory and emotional information to modulate and adapt behavioral output, which includes aggressive behavior (Gregg and Siegel, 2001). Neural inputs from the PFC to the VMHvl originate largely from the mPFC and the orbital frontal cortex (OFC) (Toth et al, 2010). Lesions of these cortical regions caused by trauma, tumors, and neurodegeneration result in emotional disturbances, including disinhibited aggressive behavior. Striking lesion examples include Phineas Gage(Damasio et al, 1994; Van Horn et al, 2012), and patients who had suffered penetrating head injuries during their service in Vietnam (Grafman et al, 1996; Pardini et al, 2011). Imaging studies further support a role of the mPFC and OFC in modulating aggressive behavior. For example, patients with borderline personality disorder or antisocial personality disorder display reductions in mPFC and OFC volumes (Hazlett et al, 2005; Narayan et al, 2007; Raine et al, 2000). Frontal activity is inversely correlated with history of violence, and impulsive aggressive behavior (Goyer et al, 1994; Lee et al, 2008; Raine et al, 1998; Volkow et al, 1995). These studies support a central role of the mPFC and OFC in serving as a top–down ‘brake’ on the hypothalamic-PAG aggression pathway.
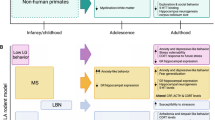
Monoaminergic modulation of aggression circuitry. The central VMHvl–PAG aggression pathway is most prominently controlled by the lateral septum (LS), the amygdala (AMY), and mPFC/OFC (a, central part). This aggression circuit in turn is modulated by 5-HT and DA signaling (a, lateral part). Red indicates aggression promoting circuit elements and green indicates aggression ameliorating circuit elements, as revealed through local lesioning, inhibition, and/or stimulation studies. The dark colors for VMHvl and LS indicate the high severity of behavioral consequences elicited by stimulation of the VMHvl (also known as the ‘hypothalamic attack area’) and septal lesioning (eliciting ‘septal rage’). (b) Summary of studies giving insight into the role of local 5-HT and DA signaling in modulating aggressive behavior. AH, anterior hypothalamic nucleus; DA, dopamine; MS, medial septum; PAGdl, dorsolateral periaqueductal grey; 5-HT, serotonin.
In summary, studies on humans and animal models have identified critical nodes within a complex circuit controlling aggressive behavior. Intriguingly, it overlaps with circuitry controlling fear, anxiety, and mating behavior at the neuroanatomical and even cellular level (Canteras and Graeff, 2014; Lin et al, 2011; Silva et al, 2013; Tye et al, 2011), indicating that the interdependence of these behaviors is hardwired.
Monoaminergic afferents target the central nodes of this aggression circuitry, and thereby confer modulatory and regulatory consequences on behavior (Figure 3). However, monoaminergic action in target regions depends on several factors ranging from the neuroanatomical connectivity map at the cellular level and the receptor complement present on the various cell types, to the intensity and pattern of the monoaminergic signal. A few studies have focused on the role of specific receptors within specific regions. PET imaging studies demonstrate a negative correlation between lifetime aggression and 5-HT1A receptor binding in the anterior cingulate, mPFC and OFC, the amygdala, and the dorsal raphe (Parsey et al, 2002). Consistently, local infusion of 5-HT1A and 5-HT1B receptor agonists into the ventral OFC decreases aggressive behavior in rodents (Centenaro et al, 2008; da Veiga et al, 2011). Local infusion of a 5-HT1A receptor agonist into median raphe nucleus, the corticomedial amygdaloid nucleus, or the dorsal PAG also reduces aggressive behavior, whereas conversely local infusion of a 5-HT1A receptor agonist into the medial septal area increases aggressive behavior (De Almeida and Lucion, 1997). In cats, local infusion of a 5-HT1A receptor agonist into the PAG decreases defensive rage behavior elicited by electrical stimulation of the medial hypothalamus (Gregg and Siegel, 2001; Shaikh et al, 1997). Finally, 5-HT1A and 5-HT1B receptors modulate aggression by suppressing vasopressin’s proaggressive function in the anterior hypothalamus (AH; Jackson et al, 2005; Morrison and Melloni, 2014). Together, these studies demonstrate a broad involvement of 5-HT1 receptor-type signaling in modulating, mostly inhibiting, aggressive behavior. Local infusion of 5-HT2A/2C receptor agonist into the corticomedial amygdaloid nucleus increases, but infusion into the dorsal PAG reduces aggression in female rats (de Almeida et al, 2005b, 2006). Interestingly, orbitofrontal availability of 5-HT2A receptors assessed through PET imaging is higher in personality disorder patients with physical aggression when compared with patients without aggression or healthy controls (Rosell et al, 2010). These findings are congruent with the role of 5-HT1-type receptor signaling, indicating that 5-HT-mediated inhibition of PFC activity (5-HT1A and 5-HT1B receptor agonism and 5-HT2A receptor antagonism) acts to decrease aggressive behavior. However, this conclusion contradicts the model in which the PFC serves as a top–down ‘brake’ on the hypothalamic-PAG aggression pathway. Reconciling these models will require higher resolution knowledge about prefrontal circuitry, taking into consideration 5-HT receptor localization, 5-HT/DA interaction, and local inhibitory and excitatory networks on one hand, and behavioral dissection along different types of aggression and impulsivity on the other hand.
PET imaging studies also give some insight into the role of DA signaling in human aggression. For example, amphetamine-associated striatal DA release is positively correlated with impulsivity (Buckholtz et al, 2010a). However, other studies indicate the opposite relationship, finding a negative correlation between levels of aggression and DA storage capacity in the midbrain and the striatum (Schluter et al, 2013). Patients suffering from schizophrenia, who display high levels of aggression, show an upregulation of the D2 receptor in the striatum (Hirvonen et al, 2005), and haloperidol and risperidone are often used successfully to alleviate aggression in such patients (Volavka et al, 2004b). In cats, apomorphine or D2 receptor agonist microinjection into the medial preoptic-AH (mPO-AH) facilitates feline affective defense behavior elicited by electric stimulation of the VMH (Sweidan et al, 1991). Conversely, D2 receptor antagonist infusion into the VMH blocks the proaggressive effect of both electrical stimulation and apomorphine potentiation (Sweidan et al, 1991). Likewise, D2 receptor antagonist infusion into the AH of an aggressive Syrian hamster model dose dependently suppressed aggressive behavior (Schwartzer and Melloni, 2010). In a rat model of aggression, local infusion of haloperidol into the NAc reduces aggressive behavior (Beiderbeck et al, 2012). Taken together, these findings provide evidence for D2 receptor mediated promotion of aggression in the mPO-AH, VMH, and NAc.
How does monoamine signaling during the PA sensitive period affect circuit properties to impose changes on aggressive behavior? Monoaminergic systems themselves remain plastic during adolescence, and thus permanent alterations to their function could underlie altered adult behavior. For example, cocaine treatment during adolescence increases aggressive behavior in male Syrian hamsters (Harrison et al, 2000), and also leads to deficits in 5-HT afferent innervations to the AH, lateral septum, medial amygdala, and BNST (DeLeon et al, 2002). Because 5-HT signaling inhibits the proaggressive effect of vasopressin infusion into the AH, and because electrically evoked vasopressin release in the AH is increased in hamsters after chronic cocaine exposure from P27 to P56 (Delville et al, 1996; Ferris et al, 1997; Ferris and Potegal, 1988; Jackson et al, 2005; Koolhaas et al, 1998), a model was put forward in which chronic PA cocaine exposure reduces 5-HTergic afferents to the AH, disinhibiting vasopressin’s proaggressive effects (Jackson et al, 2005; Morrison and Melloni, 2014). Such a model is consistent with the 5-HT hypothesis of aggression, in particular with hypo-5-HTergic animal models displaying increased aggressive behavior. Nevertheless, the causal relationships of the model remain to be proven and in the context of sensitive period conceptualization, it will be interesting to see if behavioral, anatomical, and physiological consequences are transient or persistent.
Another example suggesting a monoaminergic mechanism is based on the correlation between aggression and the behavioral response to amphetamine (Yu et al, 2014): because of the bidirectional consequences on aggression and amphetamine-induced locomotion seen with P22–P41 5-HTT and DAT blockade, it is tempting to speculate that the primary hit occurs at the DA system. In this model, PA DA and 5-HT signaling bidirectionally affect the DA system to opposingly alter aggressive behavior and behavioral response to amphetamine challenge. Alternatively, DAergic and 5-HTergic PA signaling could act on one common, or multiple independent, downstream circuits that affect aggression. Here, high aggression caused by high PA DA signaling should be rescued by concomitant high PA 5-HT signaling. However, PA MAOA blockade blocks both 5-HT and DA metabolism, yet it increases aggression and behavioral response to amphetamine (Yu et al, 2014), indicating that the DA system is downstream of and dominant over the 5-HT system. Such a model is congruent with the DA hypothesis of aggression, and in particular with the enhanced aggressive behavior observed in hyper-DAergic animal. However again, the causal relationships of this model remain to be proven.
Models of general 5-HT hypofunction or DA hyperfunction cannot explain the specificity of behavioral consequences observed with PA monoaminergic perturbations. For example, PA monoamine interference impacts adult aggressive but not affective behavior, but changes to the affective domain would be expected if a globally altered 5-HT system underlies the behavioral sequelae. Likewise, animals with altered aggression do not display altered baseline locomotor activity, which would be expected if a globally altered DA system mechanistically underlies the behavioral sequelae. Hence, it is likely that PA monoamine interference impacts only certain aspects of monoamine systems, such as anatomically defined sub-projections or specific signaling components. An example for the latter mechanism is provided by the consequences of juvenile methylphenidate exposure. Specifically, P20–P35 methylphenidate administration reduces DRD3 (but not DRD1, DRD2, DRD4, or DRD5) expression in the mPFC (but not NAc or striatum) at P60 in rats (Andersen et al, 2008), and these changes are associated with differential molecular responses to DA system targeting drugs (Andersen and Sonntag, 2014).
Lastly, many circuit elements downstream of monoaminergic synapses could carry the critical changes that are responsible for the behavioral phenotypes. For example, the functional connectivity between the central mediators and modulators of aggressive behavior, the septum, the amygdala, the PFC, the VMH, and the PAG, might be permanently established through PA monoamine signaling. The most prominent albeit still somewhat indirect examples in support of this model stem from human imaging studies. Humans carrying the MAOA-L allele, which is associated with increased aggression and impulsivity, also demonstrate aberrant coupling between the amygdala and the ventromedial PFC when exposed to emotionally relevant stimuli (Buckholtz and Meyer-Lindenberg, 2008; Dannlowski et al, 2009). Furthermore, metabolic activity between right OFC and ventral amygdala is tightly coupled in healthy subjects but not in patients with borderline personality disorder (New et al, 2007). In animal models, optogenetic tools can be applied to assess physiological connectivity and to investigate the relationship between circuit parameters such as connectivity and behavior. Therefore, these tools will have an important role in testing alternative models and in elucidating circuitry-related mechanisms.
PA Adversity and Monoamine Perturbation
Adolescence is a vulnerability window for stress exposure to elicit long-lasting behavioral consequences. Human adolescents exhibit greater stress reactivity than non-adolescents (Dahl and Gunnar, 2009) and PA stress can trigger the onset of neuropsychiatric disorders, most prominently schizophrenia and substance abuse (Hoffman and Dobscha, 1989). Furthermore, chronic stress during adolescence impacts PFC development (Casey et al, 2010, 2011; Hoftman and Lewis, 2011; Lupien et al, 2009; Selemon, 2013) and is associated with lower cognitive performance in adulthood, both in humans (Casey et al, 2010; Rahdar and Galvan, 2014) and rodents (Lukkes et al, 2009). Interestingly, although the DA system undergoes a transient expansion phase of high plasticity during adolescence (Figure 1), stress exposure can influence its maturation. Exposure to social defeat stress during adolescence, and not adulthood, for example reduces basal extracellular levels of DA in the mPFC but not NAc (Watt et al, 2009) and increases behavioral response to amphetamine challenge (Burke et al, 2013). mPFC DA release inhibits glutamatergic input to the NAc (Thierry et al, 1986), hence disinhibition of this pathway might underlie the locomotor enhancing effect. Similarly, post-weaning stress through isolation rearing decreases DA turnover in mPFC (Heidbreder et al, 2000), but enduringly increases both basal and stimulant-induced DA levels in NAc (Hall et al, 1998; Jones et al, 1992). Together, these data indicate enhanced mesolimbic and reduced mesocortical DAergic activity as a consequence of PA stress.
Although 5-HT system maturation precedes DA system maturation (Lambe et al, 2000), PA stress still impacts the 5-HT system. Isolation rearing decreases basal 5-HT turnover in the NAc (Heidbreder et al, 2000), but not in the PFC or caudate putamen (Jones et al, 1992). However, isolation rearing increases NAc 5-HT release in response to inescapable foot shock and associated context exposure (Fulford and Marsden, 1998, 2007). Conversely, isolation stress attenuates amphetamine-, KCl- and novelty-evoked 5-HT release in the PFC and HPC (Bickerdike et al, 1993; Dalley et al, 2002), whereas post-weaning social isolation and maternal separation, change 5-HT fiber density in hypothalamic areas involved in aggression (Haller, 2013).
Together, these findings demonstrate reorganization of the DAergic and 5-HTergic systems in response to PA adversity, which might impact adult behaviors including aggression.
Clinical Relevance
Aggressive behavior has a large societal impact and contributes to the pathology of a number of psychiatric conditions including psychotic disorders, anxiety disorders, attention deficit disorder, drug abuse, and suicide. Elucidating the developmental contribution to pathological aggression is central to understanding its pathophysiology, and thus critical to advance our understanding of these disorders in order to ultimately devise effective prevention and treatment strategies. Human aggressive behavior has its roots in infant development, but during childhood and adolescence control of aggressive impulses is established (Cote et al, 2007; Nagin and Tremblay, 1999; Tremblay and Szyf, 2010). Thus, there are two conceptual developmental phases for the establishment of adult aggressive behavior, (1) the establishment of baseline aggression and (2) the establishment of the control of baseline aggression. Both phases are influenced by genetic and environmental factors (Cote et al, 2007). Highlighted in this review is the latter phase, which passes through a 5-HT- and DA-sensitive period, and to the best of our knowledge impacts circuit maturation of systems such as the monoaminergic systems and the PFC, which ultimately control and modulate the central hypothalamic-PAG pathway. Findings on the 5-HT- and DA-sensitive PA period comport with human vulnerabilities to aggression conferred by functional genetic polymorphisms. For example, aggressive behavior has been associated with loss-of-function and low-expressing MAOA alleles (Brunner et al, 1993; Buckholtz and Meyer-Lindenberg, 2008; Caspi et al, 2002; Zalsman et al, 2005), the 10R variant of DAT1 (Bedard et al, 2010; Guo et al, 2007), and the low-activity met allele of the COMT (Volavka et al, 2004a). The sensitive period model predicts that these risk alleles act primarily during PA to alter brain maturation and circuit formation leading to altered behaviors.
A specific type of environmental factor relevant to the 5-HT- and DA-sensitive PA period is drug exposure. Because molecules targeting monoamine signaling constitute the most widely prescribed (eg, antidepressants/anxiolytics) and/or abused (eg, amphetamine, methamphetamine, cocaine) psychoactive drugs in the markets today, their use during adolescence might significantly impact public health, beyond their prescribed or recreational purpose. For example, SSRIs taken during PA might impact brain maturation to reduce adult aggression, whereas stimulant exposure during PA could increase adult aggressive behavior. More data is needed to understand human relevance and adequately compare risks and benefits, especially of prescribed medication, but some findings already indicate translatability. For example, chronic stimulant exposure increases aggressive behavior in rodents, non-human primates and humans (Dawe et al, 2009; Martin et al, 1990; Sokolov et al, 2004), even in abstinent stimulant users (Sekine et al, 2006). Furthermore, human individuals with antisocial traits also show mesolimbic DA hypersensitivity to amphetamine, as impulsivity is positively correlated with the magnitude of amphetamine-induced DA release in the striatum (Buckholtz et al, 2010a).
On the basis of the notion that DAergic abnormalities contribute to specific complex disorders such as schizophrenia and substance abuse, the PA 5-HT- and DA-sensitive period might have broader etiological relevance for psychopathologies beyond aggression. In this context, it is important to emphasize the bidirectional characteristic of the monoaminergic modulation of brain development and adult behavior. Thus, findings not only give insight into risk factors for psychopathology (exposure as a function of time, disrupting brain development to negatively impact adult function), but also reveal potential preventive treatment approaches (exposure as a function of time, normalizing brain development to positively impact adult function).
OUTLOOK
The available data demonstrate the existence of sensitive periods that influence life-long vulnerability to anxiety, depression, aggression, and substance abuse. Such sensitive periods have been most extensively characterized for sensory systems (eg, visual cortex), but conceptually similar principles apply to the development and organization of brain circuitry that mediate the more complex behaviors described here. Specifically, we have reviewed three sensitive periods during which transiently altered monoamine signaling carries long-lasting consequences on adult brain function.
Respective findings lead to the following three working hypotheses:
-
1
Any manipulation, which increases 5-HT signaling during development (P0–P4 in mice), will impair somatosensory cortex maturation and lateral geniculate nucleus/superior colliculus topography.
-
2
Any manipulation, which increases 5-HT signaling during development (P2–P11 in mice), will increase adult anxiety/depression-like behavior and impair cognition.
-
3
Any manipulation, which increases DA signaling during development (P22–P41 in mice), will increase adult aggression and behavioral response to stimulants, whereas any manipulation that increases 5-HT signaling during that same period will decrease adult aggression and behavioral stimulant sensitivity.
Respective findings have the following three translational implications:
-
1
Genetic and environmental factors, which affect 5-HT signaling during gestational development, act in concert to predispose to, or protect against, impaired topography within somatosensory and visual system neurocircuitry.
-
2
Genetic and environmental factors, which affect 5-HT signaling during late gestational development, act in concert to predispose to, or protect against, depression and anxiety disorders and cognitive dysfunction.
-
3
Genetic and environmental factors, which affect DA and 5-HT signaling during adolescence, act in concert to predispose to, or protect against, high aggression and DA dysfunction.
Relevance is underscored by the multitude of known rare and common functional variants in genes impacting 5-HT and DA signaling, the impact of the omnipresent environmental factor ‘stress’ on monoamine signaling, and the high frequency in the use of drugs and medications that target the 5-HT and DA systems.
Furthering our knowledge of sensitive periods that determine the developmental trajectory of complex behaviors is a necessary step toward improving prevention and treatment approaches for neuropsychiatric disorders. Preclinical studies will continue to identify neurobiological correlates of disordered behaviors and test causal relationships in current and future neuropathology models, leading the way to novel treatment approaches. Genetic studies might take advantage of the predicted converging effects of multiple genetic factors on monoaminergic signaling. Likewise, gene × environment or environment × environment interaction studies might analyze the impact of various factors that converge on specific monoamine signaling pathways. Longitudinal and population-based epidemiological studies investigating the side effects of drugs and medications years after cessation of consumption/treatment are essential.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
References
Adamec R, Burton P, Blundell J, Murphy DL, Holmes A (2006). Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res 170: 126–140.
Aguilar MA, Minarro J, Perez-Iranzo N, Simon VM (1994). Behavioral profile of raclopride in agonistic encounters between male mice. Pharmacol Biochem Behav 47: 753–756.
Alenina N, Kikic D, Todiras M, Mosienko V, Qadri F, Plehm R et al (2009). Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc Natl Acad Sci USA 106: 10332–10337.
Alvarez C, Vitalis T, Fon EA, Hanoun N, Hamon M, Seif I et al (2002). Effects of genetic depletion of monoamines on somatosensory cortical development. Neuroscience 115: 753–764.
Andersen SL (2005). Stimulants and the developing brain. Trends Pharmacol Sci 26: 237–243.
Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA Jr. (2002). Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci 5: 13–14.
Andersen SL, Napierata L, Brenhouse HC, Sonntag KC (2008). Juvenile methylphenidate modulates reward-related behaviors and cerebral blood flow by decreasing cortical D3 receptors. Eur J Neurosci 27: 2962–2972.
Andersen SL, Sonntag KC (2014). Juvenile methylphenidate reduces prefrontal cortex plasticity via D3 receptor and BDNF in adulthood. Front Synaptic Neurosci 6: 1.
Anderson AD, Oquendo MA, Parsey RV, Milak MS, Campbell C, Mann JJ (2004). Regional brain responses to serotonin in major depressive disorder. J Affect Disord 82: 411–417.
Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D et al (2008). Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol 198: 194 e191-195.
Angoa-Perez M, Kane MJ, Briggs DI, Sykes CE, Shah MM, Francescutti DM et al (2012). Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity. J Neurochem 121: 974–984.
Ansorge MS, Morelli E, Gingrich JA (2008). Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci 28: 199–207.
Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA (2004). Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 306: 879–881.
Araki KY, Sims JR, Bhide PG (2007). Dopamine receptor mRNA and protein expression in the mouse corpus striatum and cerebral cortex during pre- and postnatal development. Brain Res 1156: 31–45.
Arango V, Huang YY, Underwood MD, Mann JJ (2003). Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res 37: 375–386.
Armbruster D, Moser DA, Strobel A, Hensch T, Kirschbaum C, Lesch KP et al (2009). Serotonin transporter gene variation and stressful life events impact processing of fear and anxiety. Int J Neuropsychopharmacol 12: 393–401.
Atmaca M, Onalan E, Yildirim H, Yuce H, Koc M, Korkmaz S et al (2011). Serotonin transporter gene polymorphism implicates reduced orbito-frontal cortex in obsessive-compulsive disorder. J Anxiety Disord 25: 680–685.
Audero E, Mlinar B, Baccini G, Skachokova ZK, Corradetti R, Gross C (2013). Suppression of serotonin neuron firing increases aggression in mice. J Neurosci 33: 8678–8688.
Bakker MK, Kolling P, van den Berg PB, de Walle HE, de Jong van den Berg LT (2008). Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol 65: 600–606.
Bambico FR, Nguyen NT, Gobbi G (2009). Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur Neuropsychopharmacol 19: 215–228.
Bannai M, Fish EW, Faccidomo S, Miczek KA (2007). Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology (Berl) 193: 295–304.
Baram TZ, Davis EP, Obenaus A, Sandman CA, Small SL, Solodkin A et al (2012). Fragmentation and unpredictability of early-life experience in mental disorders. Am J Psychiatry 169: 907–915.
Barratt ES, Felthous AR (2003). Impulsive versus premeditated aggression: implications for mens rea decisions. Behav Sci Law 21: 619–630.
Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC et al (2008). Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA 105: 1333–1338.
Beaver KM, Wright JP, DeLisi M, Walsh A, Vaughn MG, Boisvert D et al (2007). A gene x gene interaction between DRD2 and DRD4 is associated with conduct disorder and antisocial behavior in males. Behav Brain Funct 3: 30.
Bedard AC, Schulz KP, Cook EH Jr., Fan J, Clerkin SM, Ivanov I et al (2010). Dopamine transporter gene variation modulates activation of striatum in youth with ADHD. Neuroimage 53: 935–942.
Beiderbeck DI, Reber SO, Havasi A, Bredewold R, Veenema AH, Neumann ID (2012). High and abnormal forms of aggression in rats with extremes in trait anxiety—involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology 37: 1969–1980.
Beique JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L et al (2004). Serotonergic regulation of membrane potential in developing rat prefrontal cortex: coordinated expression of 5-hydroxytryptamine (5-HT)1A, 5-HT2A, and 5-HT7 receptors. J Neurosci 24: 4807–4817.
Benekareddy M, Goodfellow NM, Lambe EK, Vaidya VA (2010). Enhanced function of prefrontal serotonin 5-HT(2) receptors in a rat model of psychiatric vulnerability. J Neurosci 30: 12138–12150.
Benekareddy M, Vadodaria KC, Nair AR, Vaidya VA (2011). Postnatal serotonin type 2 receptor blockade prevents the emergence of anxiety behavior, dysregulated stress-induced immediate early gene responses, and specific transcriptional changes that arise following early life stress. Biol Psychiatry 70: 1024–1032.
Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A et al (1998). Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (‘Ecstasy’) in serotonin transporter-deficient mice. Mol Pharmacol 53: 649–655.
Bennett-Clarke CA, Leslie MJ, Chiaia NL, Rhoades RW (1993). Serotonin 1B receptors in the developing somatosensory and visual cortices are located on thalamocortical axons. Proc Natl Acad Sci USA 90: 153–157.
Bennett-Clarke CA, Leslie MJ, Lane RD, Rhoades RW (1994). Effect of serotonin depletion on vibrissa-related patterns of thalamic afferents in the rat’s somatosensory cortex. J Neurosci 14: 7594–7607.
Berard A, Ramos E, Rey E, Blais L, St-Andre M, Oraichi D (2007). First trimester exposure to paroxetine and risk of cardiac malformations in infants: the importance of dosage. Birth Defects Res B Dev Reprod Toxicol 80: 18–27.
Berman ME, McCloskey MS, Fanning JR, Schumacher JA, Coccaro EF (2009). Serotonin augmentation reduces response to attack in aggressive individuals. Psychol Sci 20: 714–720.
Bhakta SG, Zhang JP, Malhotra AK (2012). The COMT Met158 allele and violence in schizophrenia: a meta-analysis. Schizophr Res 140: 192–197.
Bickerdike MJ, Wright IK, Marsden CA (1993). Social isolation attenuates rat forebrain 5-HT release induced by KCI stimulation and exposure to a novel environment. Behav Pharmacol 4: 231–236.
Blokland A, Lieben C, Deutz NE (2002). Anxiogenic and depressive-like effects, but no cognitive deficits, after repeated moderate tryptophan depletion in the rat. J Psychopharmacol 16: 39–49.
Blue ME, Erzurumlu RS, Jhaveri S (1991). A comparison of pattern formation by thalamocortical and serotonergic afferents in the rat barrel field cortex. Cerebral Cortex 1: 380–389.
Bonnin A, Goeden N, Chen K, Wilson ML, King J, Shih JC et al (2011). A transient placental source of serotonin for the fetal forebrain. Nature 472: 347–350.
Bonnin A, Peng W, Hewlett W, Levitt P (2006). Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience 141: 781–794.
Bonnin A, Torii M, Wang L, Rakic P, Levitt P (2007). Serotonin modulates the response of embryonic thalamocortical axons to netrin-1. Nature Neurosci 10: 588–597.
Bortolato M, Godar SC, Alzghoul L, Zhang J, Darling RD, Simpson KL et al (2013). Monoamine oxidase A and A/B knockout mice display autistic-like features. Int J Neuropsychopharmacol 16: 869–888.
Bradley SL, Dodelzon K, Sandhu HK, Philibert RA (2005). Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. Am J Med Genet B Neuropsychiatr Genet 136B: 58–61.
Brandon CL, Marinelli M, Baker LK, White FJ (2001). Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology 25: 651–661.
Brizer DA (1988). Psychopharmacology and the management of violent patients. Psychiatr Clin North Am 11: 551–568.
Brocke B, Armbruster D, Muller J, Hensch T, Jacob CP, Lesch KP et al (2006). Serotonin transporter gene variation impacts innate fear processing: acoustic startle response and emotional startle. Mol Psychiatry 11: 1106–1112.
Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV (2001). 3,4-methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci 21: 3228–3235.
Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE et al (1982). Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am J Psychiatry 139: 741–746.
Brown GL, Goodwin FK, Ballenger JC, Goyer PF, Major LF (1979). Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res 1: 131–139.
Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH Jr. (2006). 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry 163: 2148–2156.
Brunner D, Hen R (1997). Insights into the neurobiology of impulsive behavior from serotonin receptor knockout mice. Ann NY Acad Sci 836: 81–105.
Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA (1993). Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262: 578–580.
Buckholtz JW, Meyer-Lindenberg A (2008). MAOA and the neurogenetic architecture of human aggression. Trends Neurosci 31: 120–129.
Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R et al (2010a). Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci 13: 419–421.
Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS et al (2010b). Dopaminergic network differences in human impulsivity. Science 329: 532.
Burke AR, Forster GL, Novick AM, Roberts CL, Watt MJ (2013). Effects of adolescent social defeat on adult amphetamine-induced locomotion and corticoaccumbal dopamine release in male rats. Neuropharmacology 67: 359–369.
Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J et al (2001). Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry 158: 1126–1133.
Cannon DM, Klaver JM, Klug SA, Carlson PJ, Luckenbaugh DA, Ichise M et al (2013). Gender-specific abnormalities in the serotonin transporter system in panic disorder. Int J Neuropsychopharmacol 16: 733–743.
Canteras NS, Graeff FG (2014). Executive and modulatory neural circuits of defensive reactions: implications for panic disorder. Neurosci Biobehav Rev (http://dx.doi.org/10.1016/j.neubiorev.2014.03.020).
Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S et al (1995). Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science 268: 1763–1766.
Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P (1996). Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron 16: 297–307.
Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ et al (2010). The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev Psychobiol 52: 225–235.
Casey BJ, Ruberry EJ, Libby V, Glatt CE, Hare T, Soliman F et al (2011). Transitional and translational studies of risk for anxiety. Depress Anxiety 28: 18–28.
Casper RC, Fleisher BE, Lee-Ancajas JC, Gilles A, Gaylor E, DeBattista A et al (2003). Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 142: 402–408.
Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW et al (2002). Role of genotype in the cycle of violence in maltreated children. Science 297: 851–854.
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H et al (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301: 386–389.
Centenaro LA, Vieira K, Zimmermann N, Miczek KA, Lucion AB, de Almeida RM (2008). Social instigation and aggressive behavior in mice: role of 5-HT1A and 5-HT1B receptors in the prefrontal cortex. Psychopharmacology (Berl) 201: 237–248.
Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC et al (2013). Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci 33: 13978–13988 13988a.
Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL et al (2006). Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 354: 579–587.
Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL (1996). Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 335: 1010–1015.
Chameau P, Inta D, Vitalis T, Monyer H, Wadman WJ, van Hooft JA (2009). The N-terminal region of reelin regulates postnatal dendritic maturation of cortical pyramidal neurons. Proc Natl Acad Sci USA 106: 7227–7232.
Charney DS (1998). Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry 59 Suppl 14: 11–14.
Chen K, Holschneider DP, Wu W, Rebrin I, Shih JC (2004). A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J Biol Chem 279: 39645–39652.
Chengappa KN, Ebeling T, Kang JS, Levine J, Parepally H (1999). Clozapine reduces severe self-mutilation and aggression in psychotic patients with borderline personality disorder. J Clin Psychiatry 60: 477–484.
Chiavegatto S, Dawson VL, Mamounas LA, Koliatsos VE, Dawson TM, Nelson RJ (2001). Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc Natl Acad Sci USA 98: 1277–1281.
Coccaro EF, Berman ME, Kavoussi RJ, Hauger RL (1996). Relationship of prolactin response to d-fenfluramine to behavioral and questionnaire assessments of aggression in personality-disordered men. Biol Psychiatry 40: 157–164.
Coccaro EF, Kavoussi RJ (1997a). Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Arch Gen Psychiatry 54: 1081–1088.
Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL (1997b). Central serotonin activity and aggression: inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. Am J Psychiatry 154: 1430–1435.
Coccaro EF, Siever LJ, Klar HM, Maurer G, Cochrane K, Cooper TB et al (1989). Serotonergic studies in patients with affective and personality disorders. Correlates with suicidal and impulsive aggressive behavior. Arch Gen Psychiatry 46: 587–599.
Colzato LS, van den Wildenberg WP, Van der Does AJ, Hommel B (2010). Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience 170: 782–788.
Congdon E, Lesch KP, Canli T (2008). Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet 147B: 27–32.
Cote SM, Boivin M, Nagin DS, Japel C, Xu Q, Zoccolillo M et al (2007). The role of maternal education and nonmaternal care services in the prevention of children’s physical aggression problems. Arch Gen Psychiatry 64: 1305–1312.
Couppis MH, Kennedy CH (2008). The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology (Berl) 197: 449–456.
Crane L, Goddard L, Pring L (2009). Sensory processing in adults with autism spectrum disorders. Autism 13: 215–228.
Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V (2011). Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry 68: 1104–1112.
Culverhouse RC, Bowes L, Breslau N, Nurnberger JI Jr, Burmeister M, Fergusson DM et al (2013). Protocol for a collaborative meta-analysis of 5-HTTLPR, stress, and depression. BMC Psychiatry 13: 304.
da Veiga CP, Miczek KA, Lucion AB, de Almeida RM (2011). Social instigation and aggression in postpartum female rats: role of 5-Ht1A and 5-Ht1B receptors in the dorsal raphe nucleus and prefrontal cortex. Psychopharmacology (Berl) 213: 475–487.
Dahl RE, Gunnar MR (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol 21: 1–6.
Dai JX, Han HL, Tian M, Cao J, Xiu JB, Song NN et al (2008). Enhanced contextual fear memory in central serotonin-deficient mice. Proc Natl Acad Sci USA 105: 11981–11986.
Dalley JW, Everitt BJ, Robbins TW (2011). Impulsivity, compulsivity, and top-down cognitive control. Neuron 69: 680–694.
Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K et al (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315: 1267–1270.
Dalley JW, Theobald DE, Pereira EA, Li PM, Robbins TW (2002). Specific abnormalities in serotonin release in the prefrontal cortex of isolation-reared rats measured during behavioural performance of a task assessing visuospatial attention and impulsivity. Psychopharmacology (Berl) 164: 329–340.
Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR (1994). The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science 264: 1102–1105.
Dannlowski U, Ohrmann P, Konrad C, Domschke K, Bauer J, Kugel H et al (2009). Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int J Neuropsychopharmacol 12: 11–22.
Davidge KM, Atkinson L, Douglas L, Lee V, Shapiro S, Kennedy JL et al (2004). Association of the serotonin transporter and 5HT1Dbeta receptor genes with extreme, persistent and pervasive aggressive behaviour in children. Psychiatr Genet 14: 143–146.
Davidson RJ, Putnam KM, Larson CL (2000). Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science 289: 591–594.
Davis LL, Suris A, Lambert MT, Heimberg C, Petty F (1997). Post-traumatic stress disorder and serotonin: new directions for research and treatment. J Psychiatry Neurosci 22: 318–326.
Dawe S, Davis P, Lapworth K, McKetin R (2009). Mechanisms underlying aggressive and hostile behavior in amphetamine users. Curr Opin Psychiatr 22: 269–273.
de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA (2005a). Escalated aggressive behavior: dopamine, serotonin and GABA. Eur J Pharmacol 526: 51–64.
de Almeida RM, Giovenardi M, da Silva SP, de Oliveira VP, Stein DJ (2005b). Maternal aggression in Wistar rats: effect of 5-HT2A/2C receptor agonist and antagonist microinjected into the dorsal periaqueductal gray matter and medial septum. Braz J Med Biol Res 38: 597–602.
de Almeida RM, Giovenardi M, da Silva SP, de Oliveira VP, Stein DJ (2006). The effect of 5-HT(2a/2c) receptor agonist microinjected into central amygdaloid nucleus and median preoptic area on maternal aggressive behavior in rats. Rev Bras Psiquiatr 28: 130–134.
De Almeida RM, Lucion AB (1997). 8-OH-DPAT in the median raphe, dorsal periaqueductal gray and corticomedial amygdala nucleus decreases, but in the medial septal area it can increase maternal aggressive behavior in rats. Psychopharmacology (Berl) 134: 392–400.
de Boer SF, Koolhaas JM (2005). 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol 526: 125–139.
de Vries NK, van der Veere CN, Reijneveld SA, Bos AF (2013). Early neurological outcome of young infants exposed to selective serotonin reuptake inhibitors during pregnancy: results from the observational SMOK study. PLoS One 8: e64654.
DeLeon KR, Grimes JM, Connor DF, Melloni RH Jr. (2002). Adolescent cocaine exposure and offensive aggression: involvement of serotonin neural signaling and innervation in male Syrian hamsters. Behav Brain Res 133: 211–220.
Delville Y, Mansour KM, Ferris CF (1996). Testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus. Physiol Behav 60: 25–29.
Dewey D, Cantell M, Crawford SG (2007). Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. J Int Neuropsychol Soc 13: 246–256.
Di Chiara G, Camba R, Spano PF (1971). Evidence for inhibition by brain serotonin of mouse killing behaviour in rats. Nature 233: 272–273.
Diav-Citrin O, Shechtman S, Weinbaum D, Wajnberg R, Avgil M, Di Gianantonio E et al (2008). Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study. Br J Clin Pharmacol 66: 695–705.
Donaldson ZR, Piel DA, Santos TL, Richardson-Jones J, Leonardo ED, Beck SG et al (2014). Developmental effects of serotonin 1A autoreceptors on anxiety and social behavior. Neuropsychopharmacology 39: 291–302.
Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC (2002). Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry 59: 1011–1020.
Dorevitch A, Katz N, Zemishlany Z, Aizenberg D, Weizman A (1999). Intramuscular flunitrazepam versus intramuscular haloperidol in the emergency treatment of aggressive psychotic behavior. Am J Psychiatry 156: 142–144.
Dow-Edwards DL, Busidan Y (2001). Behavioral responses to dopamine agonists in adult rats exposed to cocaine during the preweaning period. Pharmacol Biochem Behav 70: 23–30.
El Marroun H, Jaddoe VW, Hudziak JJ, Roza SJ, Steegers EA, Hofman A et al (2012). Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Arch Gen Psychiatry 69: 706–714.
Erzurumlu RS, Gaspar P (2012). Development and critical period plasticity of the barrel cortex. Eur J Neurosci 35: 1540–1553.
Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A (2005). Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc Natl Acad Sci USA 102: 5582–5587.
Espinosa JS, Stryker MP (2012). Development and plasticity of the primary visual cortex. Neuron 75: 230–249.
Fairbanks LA, Melega WP, Jorgensen MJ, Kaplan JR, McGuire MT (2001). Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropsychopharmacology 24: 370–378.
Feder A, Skipper J, Blair JR, Buchholz K, Mathew SJ, Schwarz M et al (2011). Tryptophan depletion and emotional processing in healthy volunteers at high risk for depression. Biol Psychiatry 69: 804–807.
Fernandez SP, Gaspar P (2012). Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology 62: 144–154.
Ferrari PF, van Erp AM, Tornatzky W, Miczek KA (2003). Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. Eur J Neurosci 17: 371–378.
Ferris CF, Melloni RH Jr., Koppel G, Perry KW, Fuller RW, Delville Y (1997). Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci 17: 4331–4340.
Ferris CF, Potegal M (1988). Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav 44: 235–239.
Fisher HL, Cohen-Woods S, Hosang GM, Uher R, Powell-Smith G, Keers R et al (2012). Stressful life events and the serotonin transporter gene (5-HTT) in recurrent clinical depression. J Affect Disord 136: 189–193.
Flint J, Kendler KS (2014). The genetics of major depression. Neuron 81: 484–503.
Flory JD, Xu K, New AS, Finch T, Goldman D, Siever LJ (2007). Irritable assault and variation in the COMT gene. Psychiatr Genet 17: 344–346.
Franklin TB, Linder N, Russig H, Thony B, Mansuy IM (2011). Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS One 6: e21842.
Frodl T, Koutsouleris N, Bottlender R, Born C, Jager M, Morgenthaler M et al (2008). Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Mol Psychiatry 13: 1093–1101.
Fulford AJ, Marsden CA (1998). Conditioned release of 5-hydroxytryptamine in vivo in the nucleus accumbens following isolation-rearing in the rat. Neuroscience 83: 481–487.
Fulford AJ, Marsden CA (2007). An intact dopaminergic system is required for context-conditioned release of 5-HT in the nucleus accumbens of postweaning isolation-reared rats. Neuroscience 149: 392–400.
Fuller RW, Wong DT (1990). Serotonin uptake and serotonin uptake inhibition. Ann N Y Acad Sci 600: 68–78.
Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Langstrom B, Oreland L et al (2004). Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neurosci Lett 362: 189–192.
Gaspar P, Cases O, Maroteaux L (2003). The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 4: 1002–1012.
Giorgi O, De Montis G, Porceddu ML, Mele S, Calderini G, Toffano G et al (1987). Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Brain Res 432: 283–290.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG (1996). Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379: 606–612.
Godar SC, Bortolato M, Frau R, Dousti M, Chen K, Shih JC (2010). Maladaptive defensive behaviours in monoamine oxidase A-deficient mice. Int J Neuropsychopharmacol 14: 1195–1207.
Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D et al (1998). Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA 95: 9991–9996.
Gollan JK, Lee R, Coccaro EF (2005). Developmental psychopathology and neurobiology of aggression. Dev Psychopathol 17: 1151–1171.
Goyer PF, Andreason PJ, Semple WE, Clayton AH, King AC, Compton-Toth BA et al (1994). Positron-emission tomography and personality disorders. Neuropsychopharmacology 10: 21–28.
Grabe HJ, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K et al (2012). Moderation of adult depression by the serotonin transporter promoter variant (5-HTTLPR), childhood abuse and adult traumatic events in a general population sample. Am J Med Genet B Neuropsychiatr Genet 159B: 298–309.
Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM (1996). Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology 46: 1231–1238.
Gregg TR, Siegel A (2001). Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Progress Neuropsychopharmacol Biol Psychiatry 25: 91–140.
Griebel G, Perrault G, Sanger DJ (1997). A comparative study of the effects of selective and non-selective 5-HT2 receptor subtype antagonists in rat and mouse models of anxiety. Neuropharmacology 36: 793–802.
Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L et al (2002). Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature 416: 396–400.
Grzeskowiak LE, Gilbert AL, Morrison JL (2012). Neonatal outcomes after late-gestation exposure to selective serotonin reuptake inhibitors. J Clin Psychopharmacol 32: 615–621.
Gu Y, Yun L, Tian Y, Hu Z (2009). Association between COMT gene and Chinese male schizophrenic patients with violent behavior. Med Sci Monit 15: CR484–CR489.
Guo G, Roettger ME, Shih JC (2007). Contributions of the DAT1 and DRD2 genes to serious and violent delinquency among adolescents and young adults. Hum Genet 121: 125–136.
Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Muller J, Zeng Y et al (2007). Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. Int J Neuropsychopharmacol 10: 309–320.
Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E et al (2011). Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage 56: 881–889.
Hall FS, Wilkinson LS, Humby T, Inglis W, Kendall DA, Marsden CA et al (1998). Isolation rearing in rats: pre- and postsynaptic changes in striatal dopaminergic systems. Pharmacol Biochem Behav 59: 859–872.
Haller J (2013). The neurobiology of abnormal manifestations of aggression—a review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res Bull 93: 97–109.
Han DH, Park DB, Na C, Kee BS, Lee YS (2004). Association of aggressive behavior in Korean male schizophrenic patients with polymorphisms in the serotonin transporter promoter and catecholamine-O-methyltransferase genes. Psychiatry Res 129: 29–37.
Hanley GE, Brain U, Oberlander TF (2013). Infant developmental outcomes following prenatal exposure to antidepressants, and maternal depressed mood and positive affect. Early Hum Dev 89: 519–524.
Hansen HH, Sanchez C, Meier E (1997). Neonatal administration of the selective serotonin reuptake inhibitor Lu 10-134-C increases forced swimming-induced immobility in adult rats: a putative animal model of depression? J Pharmacol Exp Ther 283: 1333–1341.
Hansson SR, Mezey E, Hoffman BJ (1998). Serotonin transporter messenger RNA in the developing rat brain: early expression in serotonergic neurons and transient expression in non-serotonergic neurons. Neuroscience 83: 1185–1201.
Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D et al (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science 297: 400–403.
Harrison RJ, Connor DF, Nowak C, Melloni RH Jr (2000). Chronic low-dose cocaine treatment during adolescence facilitates aggression in hamsters. Physiol Behav 69: 555–562.
Hartley CA, McKenna MC, Salman R, Holmes A, Casey BJ, Phelps EA et al (2012). Serotonin transporter polyadenylation polymorphism modulates the retention of fear extinction memory. Proc Natl Acad Sci USA 109: 5493–5498.
Haydon PG, McCobb DP, Kater SB (1984). Serotonin selectively inhibits growth cone motility and synaptogenesis of specific identified neurons. Science 226: 561–564.
Haydon PG, McCobb DP, Kater SB (1987). The regulation of neurite outgrowth, growth cone motility, and electrical synaptogenesis by serotonin. J Neurobiol 18: 197–215.
Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ et al (2005). Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biol Psychiatry 58: 614–623.
Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G et al (2000). Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 100: 749–768.
Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D et al (1996). Allelic variation of human serotonin transporter gene expression. J Neurochem 66: 2621–2624.
Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D et al (2005). Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci 8: 20–21.
Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH et al (1998). Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci USA 95: 15049–15054.
Hellendall RP, Schambra UB, Liu JP, Lauder JM (1993). Prenatal expression of 5-HT1C and 5-HT2 receptors in the rat central nervous system. Exp Neurol 120: 186–201.
Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D (2003). Placental passage of antidepressant medications. Am J Psychiatry 160: 993–996.
Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B et al (2003). Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37: 233–247.
Hensch TK (2004). Critical period regulation. Annu Rev Neurosci 27: 549–579.
Hensch TK (2005). Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6: 877–888.
Hess WR (1928). Stammganglien-Reizversuche. Berichte der gesamten. Physiologie 42: 554–555.
Higley JD, King ST Jr., Hasert MF, Champoux M, Suomi SJ, Linnoila M (1996a). Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacology 14: 67–76.
Higley JD, Mehlman PT, Higley SB, Fernald B, Vickers J, Lindell SG et al (1996b). Excessive mortality in young free-ranging male nonhuman primates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Arch Gen Psychiatry 53: 537–543.
Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ et al (1996c). CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry 40: 1067–1082.
Higley JD, Mehlman PT, Taub DM, Higley SB, Suomi SJ, Vickers JH et al (1992). Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Arch Gen Psychiatry 49: 436–441.
Hirvonen J, van Erp TG, Huttunen J, Aalto S, Nagren K, Huttunen M et al (2005). Increased caudate dopamine D2 receptor availability as a genetic marker for schizophrenia. Arch Gen Psychiatry 62: 371–378.
Hodge GK, Butcher LL (1974). 5-Hydroxytryptamine correlates of isolation-induced aggression in mice. Eur J Pharmacol 28: 326–337.
Hoffman RE, Dobscha SK (1989). Cortical pruning and the development of schizophrenia: a computer model. Schizophr Bull 15: 477–490.
Hoftman GD, Lewis DA (2011). Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull 37: 493–503.
Hohmann CF, Hamon R, Batshaw ML, Coyle JT (1988). Transient postnatal elevation of serotonin levels in mouse neocortex. Brain Res 471: 163–166.
Holmes A, Murphy DL, Crawley JN (2002). Reduced aggression in mice lacking the serotonin transporter. Psychopharmacology (Berl) 161: 160–167.
Hong JP, Lee JS, Chung S, Jung J, Yoo HK, Chang SM et al (2008). New functional single nucleotide polymorphism (Ala72Ser) in the COMT gene is associated with aggressive behavior in male schizophrenia. Am J Med Genet B Neuropsychiatr Genet 147B: 658–660.
Inan M, Crair MC (2007). Development of cortical maps: perspectives from the barrel cortex. Neuroscientist 13: 49–61.
Iniguez SD, Alcantara LF, Warren BL, Riggs LM, Parise EM, Vialou V et al (2014). Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. J Neurosci 34: 1007–1021.
Iniguez SD, Warren BL, Bolanos-Guzman CA (2010). Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry 67: 1057–1066.
Jackson D, Burns R, Trksak G, Simeone B, DeLeon KR, Connor DF et al (2005). Anterior hypothalamic vasopressin modulates the aggression-stimulating effects of adolescent cocaine exposure in Syrian hamsters. Neuroscience 133: 635–646.
Jennings KA, Loder MK, Sheward WJ, Pei Q, Deacon RM, Benson MA et al (2006). Increased expression of the 5-HT transporter confers a low-anxiety phenotype linked to decreased 5-HT transmission. J Neurosci 26: 8955–8964.
Jiang X, Wang J, Luo T, Li Q (2009). Impaired hypothalamic-pituitary-adrenal axis and its feedback regulation in serotonin transporter knockout mice. Psychoneuroendocrinology 34: 317–331.
Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW (1992). Dopaminergic and serotonergic function following isolation rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav 43: 17–35.
Jung AB, Bennett JP Jr. (1996). Development of striatal dopaminergic function. III: Pre- and postnatal development of striatal and cortical mRNAs for the neurotrophin receptors trkBTK+ and trkC and their regulation by synaptic dopamine. Brain Res Dev Brain Res 94: 133–143.
Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG (2002). Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav 73: 131–140.
Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB (1988). Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol 269: 58–72.
Kalueff AV, Fox MA, Gallagher PS, Murphy DL (2007). Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav 6: 389–400.
Karg K, Burmeister M, Shedden K, Sen S (2011). The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry 68: 444–454.
Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castren E (2009). Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol 19: 97–108.
Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ et al (1996). Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther 277: 968–981.
Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ (1992). Major depression and generalized anxiety disorder. Same genes, (partly) different environments? Arch Gen Psychiatry 49: 716–722.
Kieler H, Artama M, Engeland A, Ericsson O, Furu K, Gissler M et al (2012). Selective serotonin reuptake inhibitors during pregnancy and risk of persistent pulmonary hypertension in the newborn: population based cohort study from the five Nordic countries. BMJ 344: d8012.
Killackey HP (1973). Anatomical evidence for cortical subdivisions based on vertically discrete thalamic projections from the ventral posterior nucleus to cortical barrels in the rat. Brain Res 51: 326–331.
Kim DK, Tolliver TJ, Huang SJ, Martin BJ, Andrews AM, Wichems C et al (2005). Altered serotonin synthesis, turnover and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. Neuropharmacology 49: 798–810.
Kinney GG, Vogel GW, Feng P (1997). Decreased dorsal raphe nucleus neuronal activity in adult chloral hydrate anesthetized rats following neonatal clomipramine treatment: implications for endogenous depression. Brain Res 756: 68–75.
Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S et al (2011a). A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J Neurosci 31: 2756–2768.
Kiyasova V, Gaspar P (2011b). Development of raphe serotonin neurons from specification to guidance. Eur J Neurosci 34: 1553–1562.
Knudsen EI (2004). Sensitive periods in the development of the brain and behavior. J Cogn Neurosci 16: 1412–1425.
Kobiella A, Reimold M, Ulshofer DE, Ikonomidou VN, Vollmert C, Vollstadt-Klein S et al (2011). How the serotonin transporter 5-HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Transl Psychiatry 1: e37.
Koe BK, Weissman A (1966). p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther 154: 499–516.
Koh KB, Choi EH, Lee YJ, Han M, Choi SS, Kim SW et al (2012). The relation of serotonin-related gene and COMT gene polymorphisms with criminal behavior in schizophrenic disorder. J Clin Psychiatry 73: 159–163.
Koolhaas JM, Everts H, de Ruiter AJ, de Boer SF, Bohus B (1998). Coping with stress in rats and mice: differential peptidergic modulation of the amygdala-lateral septum complex. Prog Brain Res 119: 437–448.
Kotler M, Barak P, Cohen H, Averbuch IE, Grinshpoon A, Gritsenko I et al (1999). Homicidal behavior in schizophrenia associated with a genetic polymorphism determining low catechol O-methyltransferase (COMT) activity. Am J Med Genet 88: 628–633.
Kovacic Z, Henigsberg N, Pivac N, Nedic G, Borovecki A (2008). Platelet serotonin concentration and suicidal behavior in combat related posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry 32: 544–551.
Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB (2006). Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 63: 622–629.
Kruesi MJ, Hibbs ED, Zahn TP, Keysor CS, Hamburger SD, Bartko JJ et al (1992). A 2-year prospective follow-up study of children and adolescents with disruptive behavior disorders. Prediction by cerebrospinal fluid 5-hydroxyindoleacetic acid, homovanillic acid, and autonomic measures? Arch Gen Psychiatry 49: 429–435.
Kruesi MJ, Rapoport JL, Hamburger S, Hibbs E, Potter WZ, Lenane M et al (1990). Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disruptive behavior disorders of children and adolescents. Arch Gen Psychiatry 47: 419–426.
Kruk MR, Vanderpoel AM, Devosfrerichs TP (1979). Induction of aggressive-behavior by electrical-stimulation in the hypothalamus of male-rats. Behaviour 70: 292–322.
Kruk MR, Vanderpoel AM, Meelis W, Hermans J, Mostert PG, Mos J et al (1983). Discriminant-analysis of the localization of aggression-inducing electrode placements in the hypothalamus of male-rats. Brain Res 260: 61–79.
Lachman HM, Nolan KA, Mohr P, Saito T, Volavka J (1998). Association between catechol O-methyltransferase genotype and violence in schizophrenia and schizoaffective disorder. Am J Psychiatry 155: 835–837.
Lambe EK, Krimer LS, Goldman-Rakic PS (2000). Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci 20: 8780–8787.
Lammers JH, Kruk MR, Meelis W, van der Poel AM (1988). Hypothalamic substrates for brain stimulation-induced attack, teeth-chattering and social grooming in the rat. Brain Res 449: 311–327.
Lauder JM (1990). Ontogeny of the serotonergic system in the rat: serotonin as a developmental signal. Ann N Y Acad Sci 600: 297–313.
Lauder JM, Bloom FE (1974). Ontogeny of monoamine neurons in the locus coeruleus, Raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol 155: 469–481.
Laurent A, Goaillard JM, Cases O, Lebrand C, Gaspar P, Ropert N (2002). Activity-dependent presynaptic effect of serotonin 1B receptors on the somatosensory thalamocortical transmission in neonatal mice. J Neurosci 22: 886–900.
Lebrand C, Cases O, Adelbrecht C, Doye A, Alvarez C, El Mestikawy S et al (1996). Transient uptake and storage of serotonin in developing thalamic neurons. Neuron 17: 823–835.
Lebrand C, Cases O, Wehrle R, Blakely RD, Edwards RH, Gaspar P (1998). Transient developmental expression of monoamine transporters in the rodent forebrain. J Comp Neurol 401: 506–524.
Lebrand C, Gaspar P, Nicolas D, Hornung JP (2006). Transitory uptake of serotonin in the developing sensory pathways of the common marmoset. J Comp Neurol 499: 677–689.
Lee TM, Chan SC, Raine A (2008). Strong limbic and weak frontal activation to aggressive stimuli in spouse abusers. Mol Psychiatry 13: 655–656.
Lehmann J, Pryce CR, Bettschen D, Feldon J (1999). The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav 64: 705–715.
Lemogne C, Gorwood P, Boni C, Pessiglione M, Lehericy S, Fossati P (2011). Cognitive appraisal and life stress moderate the effects of the 5-HTTLPR polymorphism on amygdala reactivity. Hum Brain Mapp 32: 1856–1867.
Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD et al (2003). Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 23: 8788–8799.
Lenox RH, Newhouse PA, Creelman WL, Whitaker TM (1992). Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry 53: 47–52.
Lerner Y, Lwow E, Levitin A, Belmaker RH (1979). Acute high-dose parenteral haloperidol treatment of psychosis. Am J Psychiatry 136: 1061–1064.
Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S et al (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274: 1527–1531.
Levallois C, Valence C, Baldet P, Privat A (1997). Morphological and morphometric analysis of serotonin-containing neurons in primary dissociated cultures of human rhombencephalon: a study of development. Brain Res Dev Brain Res 99: 243–252.
Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J (2009). Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology 56: 692–701.
Li H, Fertuzinhos S, Mohns E, Hnasko TS, Verhage M, Edwards R et al (2013). Laminar and columnar development of barrel cortex relies on thalamocortical neurotransmission. Neuron 79: 970–986.
Lidov HG, Molliver ME (1982a). An immunohistochemical study of serotonin neuron development in the rat: ascending pathways and terminal fields. Brain Res Bull 8: 389–430.
Lidov HG, Molliver ME (1982b). Immunohistochemical study of the development of serotonergic neurons in the rat CNS. Brain Res Bull 9: 559–604.
Lim JE, Papp A, Pinsonneault J, Sadee W, Saffen D (2006). Allelic expression of serotonin transporter (SERT) mRNA in human pons: lack of correlation with the polymorphism SERTLPR. Mol Psychiatry 11: 649–662.
Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P et al (2011). Functional identification of an aggression locus in the mouse hypothalamus. Nature 470: 221–226.
Linnoila M, Virkkunen M, Scheinin M, Nuutila A, Rimon R, Goodwin FK (1983). Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Sci 33: 2609–2614.
Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH et al (2003). Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiatry 54: 960–971.
Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M et al (2014). Dorsal Raphe neurons signal reward through 5-HT and glutamate. Neuron 81: 1360–1374.
Lo Iacono L, Gross C (2008). Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. J Neurosci 28: 6250–6257.
Loughhead AM, Fisher AD, Newport DJ, Ritchie JC, Owens MJ, DeVane CL et al (2006). Antidepressants in amniotic fluid: another route of fetal exposure. Am J Psychiatry 163: 145–147.
Lukkes JL, Mokin MV, Scholl JL, Forster GL (2009). Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm Behav 55: 248–256.
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10: 434–445.
Maciag D, Simpson KL, Coppinger D, Lu Y, Wang Y, Lin RC et al (2006). Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology 31: 47–57.
Maeda H, Maki S (1986). Dopaminergic facilitation of recovery from amygdaloid lesions which affect hypothalamic defensive attack in cats. Brain Res 363: 135–140.
Maeda H, Sato T, Maki S (1985). Effects of dopamine agonists on hypothalamic defensive attack in cats. Physiol Behav 35: 89–92.
Mague SD, Andersen SL, Carlezon WA Jr. (2005). Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry 57: 120–125.
Malison RT, Price LH, Berman R, van Dyck CH, Pelton GH, Carpenter L et al (1998). Reduced brain serotonin transporter availability in major depression as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 44: 1090–1098.
Malm H, Artama M, Brown AS, Gissler M, Gyllenberg D, Hinkka-Yli-Salomaki S et al (2012). Infant and childhood neurodevelopmental outcomes following prenatal exposure to selective serotonin reuptake inhibitors: overview and design of a Finnish Register-Based Study (FinESSI). BMC Psychiatry 12: 217.
Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF (1998). Aggression, impulsivity, and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology 19: 287–299.
Marmolejo N, Paez J, Levitt JB, Jones LB (2012). Early postnatal lesion of the medial dorsal nucleus leads to loss of dendrites and spines in adult prefrontal cortex. Dev Neurosci 34: 463–476.
Maron E, Kuikka JT, Shlik J, Vasar V, Vanninen E, Tiihonen J (2004). Reduced brain serotonin transporter binding in patients with panic disorder. Psychiatry Res 132: 173–181.
Martin SP, Smith EO, Byrd LD (1990). Effects of dominance rank on d-amphetamine-induced increases in aggression. Pharmacol Biochem Behav 37: 493–496.
Mathews TA, Fedele DE, Coppelli FM, Avila AM, Murphy DL, Andrews AM (2004). Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. J Neurosci Methods 140: 169–181.
McCarthy D, Lueras P, Bhide PG (2007). Elevated dopamine levels during gestation produce region-specific decreases in neurogenesis and subtle deficits in neuronal numbers. Brain Res 1182: 11–25.
McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M (2012). Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol 108: 1620–1630.
McCutcheon JE, Marinelli M (2009). Age matters. Eur J Neurosci 29: 997–1014.
Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J et al (1994). Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry 151: 1485–1491.
Meloy JR (2006). Empirical basis and forensic application of affective and predatory violence. Aust N Z J Psychiatry 40: 539–547.
Miczek KA, Maxson SC, Fish EW, Faccidomo S (2001). Aggressive behavioral phenotypes in mice. Behav Brain Res 125: 167–181.
Miczek KA, Mos J, Olivier B (1989). Brain 5-HT and inhibition of aggressive behavior in animals: 5-HIAA and receptor subtypes. Psychopharmacol Bull 25: 399–403.
Mik HM, Ehtesham S, Baldassarra L, De Luca V, Davidge K, Bender D et al (2007). Serotonin system genes and childhood-onset aggression. Psychiatr Genet 17: 11.
Misri S, Reebye P, Kendrick K, Carter D, Ryan D, Grunau RE et al (2006). Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry 163: 1026–1032.
Moffitt TE, Caspi A, Harrington H, Milne BJ, Melchior M, Goldberg D et al (2007). Generalized anxiety disorder and depression: childhood risk factors in a birth cohort followed to age 32. Psychol Med 37: 441–452.
Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E et al (2000). Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res 119: 251–257.
Mooney RD, Crnko-Hoppenjans TA, Ke M, Bennett-Clarke CA, Lane RD, Chiaia NL et al (1998). Augmentation of serotonin in the developing superior colliculus alters the normal development of the uncrossed retinotectal projection. J Comp Neurol 393: 84–92.
Mooney RD, Shi MY, Rhoades RW (1994). Modulation of retinotectal transmission by presynaptic 5-HT1B receptors in the superior colliculus of the adult hamster. J Neurophysiol 72: 3–13.
Morelli E, Moore H, Rebello TJ, Gray N, Steele K, Esposito E et al (2011). Chronic 5-HT transporter blockade reduces DA signaling to elicit basal ganglia dysfunction. J Neurosci 31: 15742–15750.
Morford LL, Inman-Wood SL, Gudelsky GA, Williams MT, Vorhees CV (2002). Impaired spatial and sequential learning in rats treated neonatally with D-fenfluramine. Eur J Neurosci 16: 491–500.
Morrison TR, Melloni RH Jr. (2014). The role of serotonin, vasopressin, and serotonin/vasopressin interactions in aggressive behavior. Curr Top Behav Neurosci 17: 189–228.
Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M et al (2012). Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl Psychiatry 2: e122.
Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL et al (2009). Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav 8: 129–142.
Muller JM, Morelli E, Ansorge M, Gingrich JA (2011). Serotonin transporter deficient mice are vulnerable to escape deficits following inescapable shocks. Genes Brain Behav 10: 166–175.
Munafo MR, Durrant C, Lewis G, Flint J (2009). Gene X environment interactions at the serotonin transporter locus. Biol Psychiatry 65: 211–219.
Murphy SE, Norbury R, Godlewska BR, Cowen PJ, Mannie ZM, Harmer CJ et al (2013). The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Mol Psychiatry 18: 512–520.
Murthy NV, Selvaraj S, Cowen PJ, Bhagwagar Z, Riedel WJ, Peers P et al (2010). Serotonin transporter polymorphisms (SLC6A4 insertion/deletion and rs25531) do not affect the availability of 5-HTT to [11C] DASB binding in the living human brain. Neuroimage 52: 50–54.
Nagin D, Tremblay RE (1999). Trajectories of boys’ physical aggression, opposition, and hyperactivity on the path to physically violent and nonviolent juvenile delinquency. Child Dev 70: 1181–1196.
Narayan VM, Narr KL, Kumari V, Woods RP, Thompson PM, Toga AW et al (2007). Regional cortical thinning in subjects with violent antisocial personality disorder or schizophrenia. Am J Psychiatry 164: 1418–1427.
Narboux-Neme N, Pavone LM, Avallone L, Zhuang X, Gaspar P (2008). Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs). Neuropharmacology 55: 994–1005.
Narboux-Neme N, Sagne C, Doly S, Diaz SL, Martin CB, Angenard G et al (2011). Severe serotonin depletion after conditional deletion of the vesicular monoamine transporter 2 gene in serotonin neurons: neural and behavioral consequences. Neuropsychopharmacology 36: 2538–2550.
New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R et al (2007). Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology 32: 1629–1640.
Noisin EL, Thomas WE (1988). Ontogeny of dopaminergic function in the rat midbrain tegmentum, corpus striatum and frontal cortex. Brain Res 469: 241–252.
Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman JL, Cameron HA et al (2008). Effects of adolescent fluoxetine treatment on fear-, anxiety- or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology (Berl) 200: 413–424.
Nulman I, Rovet J, Stewart DE, Wolpin J, Gardner HA, Theis JG et al (1997). Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med 336: 258–262.
Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S et al (2002). Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 159: 1889–1895.
Oberlander TF, Bonaguro RJ, Misri S, Papsdorf M, Ross CJ, Simpson EM (2008). Infant serotonin transporter (SLC6A4) promoter genotype is associated with adverse neonatal outcomes after prenatal exposure to serotonin reuptake inhibitor medications. Mol Psychiatry 13: 65–73.
Oberlander TF, Papsdorf M, Brain UM, Misri S, Ross C, Grunau RE (2010). Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med 164: 444–451.
Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE (2007). Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med 161: 22–29.
Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C (2006). Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry 63: 898–906.
Ohta K, Miki T, Warita K, Suzuki S, Kusaka T, Yakura T et al (2014). Prolonged maternal separation disturbs the serotonergic system during early brain development. Int J Dev Neurosci 33: 15–21.
Olivier B, Mos J, van Oorschot R, Hen R (1995). Serotonin receptors and animal models of aggressive behavior. Pharmacopsychiatry 28 Suppl 2: 80–90.
Olson L, Seiger A (1972). Early prenatal ontogeny of central monoamine neurons in the rat: fluorescence histochemical observations. Z Anat Entwickl 137: 301–316.
Oquendo MA, Hastings RS, Huang YY, Simpson N, Ogden RT, Hu XZ et al (2007). Brain serotonin transporter binding in depressed patients with bipolar disorder using positron emission tomography. Arch Gen Psychiatry 64: 201–208.
Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM (2009). Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J Neurosci 29: 6229–6233.
Page DT, Kuti OJ, Prestia C, Sur M (2009). Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci USA 106: 1989–1994.
Pardini M, Krueger F, Hodgkinson C, Raymont V, Ferrier C, Goldman D et al (2011). Prefrontal cortex lesions and MAO-A modulate aggression in penetrating traumatic brain injury. Neurology 76: 1038–1045.
Parks CL, Robinson PS, Sibille E, Shenk T, Toth M (1998). Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA 95: 10734–10739.
Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, Huang YY et al (2006). Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry 163: 48–51.
Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V et al (2002). Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res 954: 173–182.
Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M, Sihn S et al (1993). Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac). JAMA 269: 2246–2248.
Patkar AA, Berrettini WH, Hoehe M, Thornton CC, Gottheil E, Hill K et al (2002). Serotonin transporter polymorphisms and measures of impulsivity, aggression, and sensation seeking among African-American cocaine-dependent individuals. Psychiatry Res 110: 103–115.
Pavlov KA, Chistiakov DA, Chekhonin VP (2012). Genetic determinants of aggression and impulsivity in humans. J Appl Genet 53: 61–82.
Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH (2009). Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ 339: b3569.
Perez-Rodriguez MM, Weinstein S, New AS, Bevilacqua L, Yuan Q, Zhou Z et al (2010). Tryptophan-hydroxylase 2 haplotype association with borderline personality disorder and aggression in a sample of patients with personality disorders and healthy controls. J Psychiatric Res 44: 1075–1081.
Persico AM, Mengual E, Moessner R, Hall FS, Revay RS, Sora I et al (2001). Barrel pattern formation requires serotonin uptake by thalamocortical afferents, and not vesicular monoamine release. J Neurosci 21: 6862–6873.
Petersen CC (2007). The functional organization of the barrel cortex. Neuron 56: 339–355.
Peyrot WJ, Middeldorp CM, Jansen R, Smit JH, de Geus EJ, Hottenga JJ et al (2013). Strong effects of environmental factors on prevalence and course of major depressive disorder are not moderated by 5-HTTLPR polymorphisms in a large Dutch sample. J Affect Disord 146: 91–99.
Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS et al (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 8: 828–834.
Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A (2008). The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am J Med Genet B Neuropsychiatr Genet 147B: 543–549.
Piek JP, Dyck MJ (2004). Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Hum Mov Sci 23: 475–488.
Pietrek C, Elbert T, Weierstall R, Muller O, Rockstroh B (2013). Childhood adversities in relation to psychiatric disorders. Psychiatry Res 206: 103–110.
Popa D, Lena C, Alexandre C, Adrien J (2008). Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci 28: 3546–3554.
Popolo M, McCarthy DM, Bhide PG (2004). Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci 26: 229–244.
Potegal M, Gibbons JL, Glusman M (1980). Inhibition of muricide by septal stimulation in rats. Physiol Behav 5: 863–867.
Prater KE, Hosanagar A, Klumpp H, Angstadt M, Phan KL (2013). Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety 30: 234–241.
Preuss UW, Soyka M, Bahlmann M, Wenzel K, Behrens S, de Jonge S et al (2000). Serotonin transporter gene regulatory region polymorphism (5-HTTLPR), [3H]paroxetine binding in healthy control subjects and alcohol-dependent patients and their relationships to impulsivity. Psychiatry Res 96: 51–61.
Quinn JJ, Skipper RA, Claflin DI (2013). Infant stress exposure produces persistent enhancement of fear learning across development. Dev Psychobiol 56: 1008–1016.
Raftogianni A, Diamantopoulou A, Alikaridis F, Stamatakis A, Stylianopoulou F (2012). Effects of interaction of an early experience of reward through maternal contact or its denial with social stress during adolescence on the serotonergic system and the stress responsiveness of adult female rats. Neuroscience 209: 84–96.
Rahdar A, Galvan A (2014). The cognitive and neurobiological effects of daily stress in adolescents. Neuroimage 92C: 267–273.
Rai D, Lee BK, Dalman C, Golding J, Lewis G, Magnusson C (2013). Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ 346: f2059.
Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P (2000). Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry 57: 119–127.
Raine A, Meloy JR, Bihrle S, Stoddard J, LaCasse L, Buchsbaum MS (1998). Reduced prefrontal and increased subcortical brain functioning assessed using positron emission tomography in predatory and affective murderers. Behav Sci Law 16: 319–332.
Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M et al (1998). Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA 95: 14476–14481.
Rampono J, Proud S, Hackett LP, Kristensen JH, Ilett KF (2004). A pilot study of newer antidepressant concentrations in cord and maternal serum and possible effects in the neonate. Int J Neuropsychopharmacol 7: 329–334.
Rebello TJ, Yu Q, Goodfellow NM, Caffrey Cagliostro MK, Teissier A, Morelli E et al (2014). Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. J Neurosci 34: 12379–12393.
Reese BE, Cowey A (1983). Projection lines and the ipsilateral retino-geniculate pathway in the hooded rat. Neuroscience 10: 1233–1247.
Rentesi G, Antoniou K, Marselos M, Syrrou M, Papadopoulou-Daifoti Z, Konstandi M (2013). Early maternal deprivation-induced modifications in the neurobiological, neurochemical and behavioral profile of adult rats. Behav Brain Res 244: 29–37.
Reuter M, Kuepper Y, Hennig J (2007). Association between a polymorphism in the promoter region of the TPH2 gene and the personality trait of harm avoidance. Int J Neuropsychopharmacol 10: 401–404.
Rhoades RW, Bennett-Clarke CA, Shi MY, Mooney RD (1994). Effects of 5-HT on thalamocortical synaptic transmission in the developing rat. J Neurophysiol 72: 2438–2450.
Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A et al (2011). Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci 31: 6008–6018.
Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J et al (2009). Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA 301: 2462–2471.
Rocca P, Marchiaro L, Cocuzza E, Bogetto F (2002). Treatment of borderline personality disorder with risperidone. J Clin Psychiatry 63: 241–244.
Rodriguez-Arias M, Minarro J, Aguilar MA, Pinazo J, Simon VM (1998). Effects of risperidone and SCH 23390 on isolation-induced aggression in male mice. Eur Neuropsychopharmacol 8: 95–103.
Rodriguiz RM, Chu R, Caron MG, Wetsel WC (2004). Aberrant responses in social interaction of dopamine transporter knockout mice. Behavl Brain Res 148: 185–198.
Roeling TA, Veening JG, Kruk MR, Peters JP, Vermelis ME, Nieuwenhuys R (1994). Efferent connections of the hypothalamic ‘aggression area’ in the rat. Neuroscience 59: 1001–1024.
Rogers SJ, Ozonoff S (2005). Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry 46: 1255–1268.
Roiser JP, de Martino B, Tan GC, Kumaran D, Seymour B, Wood NW et al (2009). A genetically mediated bias in decision making driven by failure of amygdala control. J Neurosci 29: 5985–5991.
Rosell DR, Thompson JL, Slifstein M, Xu X, Frankle WG, New AS et al (2010). Increased serotonin 2A receptor availability in the orbitofrontal cortex of physically aggressive personality disordered patients. Biol Psychiatry 67: 1154–1162.
Rumajogee P, Verge D, Hanoun N, Brisorgueil MJ, Hen R, Lesch KP et al (2004). Adaption of the serotoninergic neuronal phenotype in the absence of 5-HT autoreceptors or the 5-HT transporter: involvement of BDNF and cAMP. Eur J Neurosci 19: 937–944.
Sabol SZ, Hu S, Hamer D (1998). A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 103: 273–279.
Salichon N, Gaspar P, Upton AL, Picaud S, Hanoun N, Hamon M et al (2001). Excessive activation of serotonin (5-HT) 1B receptors disrupts the formation of sensory maps in monoamine oxidase a and 5-ht transporter knock-out mice. J Neurosci 21: 884–896.
Salisbury AL, Wisner KL, Pearlstein T, Battle CL, Stroud L, Lester BM (2011). Newborn neurobehavioral patterns are differentially related to prenatal maternal major depressive disorder and serotonin reuptake inhibitor treatment. Depress Anxiety 28: 1008–1019.
Sarkar A, Chachra P, Vaidya VA (2013). Postnatal fluoxetine-evoked anxiety is prevented by concomitant 5-HT receptor blockade and mimicked by postnatal 5-HT receptor stimulation. Biol Psychiatry (in press).
Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L et al (1994). Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265: 1875–1878.
Scharinger C, Rabl U, Sitte HH, Pezawas L (2010). Imaging genetics of mood disorders. Neuroimage 53: 810–821.
Schinka JA, Busch RM, Robichaux-Keene N (2004). A meta-analysis of the association between the serotonin transporter gene polymorphism (5-HTTLPR) and trait anxiety. Mol Psychiatry 9: 197–202.
Schluter T, Winz O, Henkel K, Prinz S, Rademacher L, Schmaljohann J et al (2013). The impact of dopamine on aggression: an [18F]-FDOPA PET Study in healthy males. J Neurosci 33: 16889–16896.
Schmidt LG, Sander T, Kuhn S, Smolka M, Rommelspacher H, Samochowiec J et al (2000). Different allele distribution of a regulatory MAOA gene promoter polymorphism in antisocial and anxious-depressive alcoholics. J Neural Transm 107: 681–689.
Schmitz A, Kirsch P, Reuter M, Alexander N, Kozyra E, Kuepper Y et al (2009). The 5-HT1A C(-1019)G polymorphism, personality and electrodermal reactivity in a reward/punishment paradigm. Int J Neuropsychopharmacol 12: 383–392.
Schulz SC, Camlin KL, Berry SA, Jesberger JA (1999). Olanzapine safety and efficacy in patients with borderline personality disorder and comorbid dysthymia. Biol Psychiatry 46: 1429–1435.
Schwartzer JJ, Melloni RH Jr. (2010). Anterior hypothalamic dopamine D2 receptors modulate adolescent anabolic/androgenic steroid-induced offensive aggression in the Syrian hamster. Behav Pharmacol 21: 314–322.
Scott AL, Bortolato M, Chen K, Shih JC (2008). Novel monoamine oxidase A knock out mice with human-like spontaneous mutation. Neuroreport 19: 739–743.
Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M et al (2006). Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry 63: 90–100.
Selemon LD (2013). A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry 3: e238.
Sen S, Burmeister M, Ghosh D (2004). Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet 127B: 85–89.
Seo D, Patrick CJ, Kennealy PJ (2008). Role of serotonin and dopamine system interactions in the neurobiology of impulsive aggression and its comorbidity with other clinical disorders. Aggress Violent Behav 13: 383–395.
Shah MP, Wang F, Kalmar JH, Chepenik LG, Tie K, Pittman B et al (2009). Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacology 34: 1301–1310.
Shaikh MB, De Lanerolle NC, Siegel A (1997). Serotonin 5-HT1A and 5-HT2/1C receptors in the midbrain periaqueductal gray differentially modulate defensive rage behavior elicited from the medial hypothalamus of the cat. Brain Res 765: 198–207.
Shatz CJ, Stryker MP (1988). Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science 242: 87–89.
Siegel A, Shaikh MB (1997). The neural bases of aggression and rage in the cat. Aggress Violent Beh 2: 241–271.
Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA et al (1999). d,l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology 20: 413–423.
Sijbesma H, Schipper J, de Kloet ER, Mos J, van Aken H, Olivier B (1991). Postsynaptic 5-HT1 receptors and offensive aggression in rats: a combined behavioural and autoradiographic study with eltoprazine. Pharmacol Biochem Behav 38: 447–458.
Silva BA, Mattucci C, Krzywkowski P, Murana E, Illarionova A, Grinevich V et al (2013). Independent hypothalamic circuits for social and predator fear. Nat Neurosci 16: 1731–1733.
Simon GE, Cunningham ML, Davis RL (2002). Outcomes of prenatal antidepressant exposure. Am J Psychiatry 159: 2055–2061.
Singh JP, Volavka J, Czobor P, Van Dorn RA (2012). A meta-analysis of the Val158Met COMT polymorphism and violent behavior in schizophrenia. PLoS One 7: e43423.
Smit-Rigter LA, Noorlander CW, von Oerthel L, Chameau P, Smidt MP, van Hooft JA (2012). Prenatal fluoxetine exposure induces life-long serotonin 5-HT(3) receptor-dependent cortical abnormalities and anxiety-like behaviour. Neuropharmacology 62: 865–870.
Smit-Rigter LA, Wadman WJ, van Hooft JA (2011). Alterations in apical dendrite bundling in the somatosensory cortex of 5-HT(3A) receptor knockout mice. Front Neuroanat 5: 64.
Soderstrom H, Blennow K, Manhem A, Forsman A (2001). CSF studies in violent offenders. I. 5-HIAA as a negative and HVA as a positive predictor of psychopathy. J Neural Transm 108: 869–878.
Sodetz FJ, Bunnell BN (1970). Septal ablation and the social behavior of the golden hamster. Physiol Behav 5: 79–88.
Sodhi MS, Sanders-Bush E (2004). Serotonin and brain development. Int Rev Neurobiol 59: 111–174.
Sokolov BP, Cadet JL (2006). Methamphetamine causes alterations in the MAP kinase-related pathways in the brains of mice that display increased aggressiveness. Neuropsychopharmacology 31: 956–966.
Sokolov BP, Schindler CW, Cadet JL (2004). Chronic methamphetamine increases fighting in mice. Pharmacol Biochem Behav 77: 319–326.
Soloff PH, Price JC, Meltzer CC, Fabio A, Frank GK, Kaye WH (2007). 5HT2A receptor binding is increased in borderline personality disorder. Biol Psychiatry 62: 580–587.
Spivak B, Vered Y, Graff E, Blum I, Mester R, Weizman A (1999). Low platelet-poor plasma concentrations of serotonin in patients with combat-related posttraumatic stress disorder. Biol Psychiatry 45: 840–845.
Sretavan DW, Shatz CJ (1986). Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers within the cat's lateral geniculate nucleus. J Neurosci 6: 234–251.
Stoltenberg SF, Twitchell GR, Hanna GL, Cook EH, Fitzgerald HE, Zucker RA et al (2002). Serotonin transporter promoter polymorphism, peripheral indexes of serotonin function, and personality measures in families with alcoholism. Am J Med Genet 114: 230–234.
Strous RD, Nolan KA, Lapidus R, Diaz L, Saito T, Lachman HM (2003). Aggressive behavior in schizophrenia is associated with the low enzyme activity COMT polymorphism: a replication study. Am J Med Genet B Neuropsychiatr Genet 120B: 29–34.
Sundstrom E, Kolare S, Souverbie F, Samuelsson EB, Pschera H, Lunell NO et al (1993). Neurochemical differentiation of human bulbospinal monoaminergic neurons during the first trimester. Brain Res Dev Brain Res 75: 1–12.
Suri D, Veenit V, Sarkar A, Thiagarajan D, Kumar A, Nestler EJ et al (2013). Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol Psychiatry 73: 658–666.
Sweidan S, Edinger H, Siegel A (1991). D2 dopamine receptor-mediated mechanisms in the medial preoptic-anterior hypothalamus regulate effective defense behavior in the cat. Brain Res 549: 127–137.
Tadic A, Rujescu D, Szegedi A, Giegling I, Singer P, Moller HJ et al (2003). Association of a MAOA gene variant with generalized anxiety disorder, but not with panic disorder or major depression. Am J Med Genet B Neuropsychiatr Genet 117B: 1–6.
Takahashi H, Nakashima S, Ohama E, Takeda S, Ikuta F (1986). Distribution of serotonin-containing cell bodies in the brainstem of the human fetus determined with immunohistochemistry using antiserotonin serum. Brain Dev 8: 355–365.
Tarazi FI, Tomasini EC, Baldessarini RJ (1998a). Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci Lett 254: 21–24.
Tarazi FI, Tomasini EC, Baldessarini RJ (1998b). Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Brain Res Dev Brain Res 110: 227–233.
Teicher MH, Andersen SL, Hostetter JC Jr. (1995). Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res 89: 167–172.
Thierry AM, Le Douarin C, Penit J, Ferron A, Glowinski J (1986). Variation in the ability of neuroleptics to block the inhibitory influence of dopaminergic neurons on the activity of cells in the rat prefrontal cortex. Brain Res Bull 16: 155–160.
Thompson I, Holt C (1989). Effects of intraocular tetrodotoxin on the development of the retinocollicular pathway in the Syrian hamster. J Comp Neurol 282: 371–388.
Tidey JW, Miczek KA (1996). Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res 721: 140–149.
Toda T, Homma D, Tokuoka H, Hayakawa I, Sugimoto Y, Ichinose H et al (2013). Birth regulates the initiation of sensory map formation through serotonin signaling. Dev Cell 27: 32–46.
Toth M, Fuzesi T, Halasz J, Tulogdi A, Haller J (2010). Neural inputs of the hypothalamic ‘aggression area’ in the rat. Behav Brain Res 215: 7–20.
Tremblay RE, Szyf M (2010). Developmental origins of chronic physical aggression and epigenetics. Epigenomics 2: 495–499.
Tsang D, Ho KP, Wen HL (1986). Ontogenesis of multiple forms of monoamine oxidase in rat brain regions and liver. Dev Neurosci 8: 243–250.
Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H et al (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471: 358–362.
Underwood MD, Kassir SA, Bakalian MJ, Galfalvy H, Mann JJ, Arango V (2012). Neuron density and serotonin receptor binding in prefrontal cortex in suicide. Int J Neuropsychopharmacol 15: 435–447.
Upton AL, Ravary A, Salichon N, Moessner R, Lesch KP, Hen R et al (2002). Lack of 5-HT(1B) receptor and of serotonin transporter have different effects on the segregation of retinal axons in the lateral geniculate nucleus compared to the superior colliculus. Neuroscience 111: 597–610.
Upton AL, Salichon N, Lebrand C, Ravary A, Blakely R, Seif I et al (1999). Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: possible role of 5-HT uptake in retinal ganglion cells during development. J Neurosci 19: 7007–7024.
van Erp AM, Miczek KA (2000). Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J Neurosci 20: 9320–9325.
Van Horn JD, Irimia A, Torgerson CM, Chambers MC, Kikinis R, Toga AW (2012). Mapping connectivity damage in the case of Phineas Gage. PLoS One 7: e37454.
van Kleef ES, Gaspar P, Bonnin A (2012). Insights into the complex influence of 5-HT signaling on thalamocortical axonal system development. Eur J Neurosci 35: 1563–1572.
Verney C, Lebrand C, Gaspar P (2002). Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec 267: 87–93.
Vinkers CH, Oosting RS, van Bogaert MJ, Olivier B, Groenink L (2010). Early-life blockade of 5-HT(1A) receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biol Psychiatry 67: 309–316.
Virkkunen M, Goldman D, Nielsen DA, Linnoila M (1995). Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci 20: 271–275.
Virkkunen M, Rawlings R, Tokola R, Poland RE, Guidotti A, Nemeroff C et al (1994). CSF biochemistries, glucose metabolism, and diurnal activity rhythms in alcoholic, violent offenders, fire setters, and healthy volunteers. Arch Gen Psychiatry 51: 20–27.
Vitalis T, Ansorge MS, Dayer AG (2013). Serotonin homeostasis and serotonin receptors as actors of cortical construction: special attention to the 5-HT3A and 5-HT6 receptor subtypes. Front Cell Neurosci 7: 93.
Vitalis T, Cases O, Callebert J, Launay JM, Price DJ, Seif I et al (1998). Effects of monoamine oxidase A inhibition on barrel formation in the mouse somatosensory cortex: determination of a sensitive developmental period. J Comp Neurol 393: 169–184.
Vogel G, Neill D, Hagler M, Kors D (1990). A new animal model of endogenous depression: a summary of present findings. Neurosci Biobehav Rev 14: 85–91.
Volavka J, Bilder R, Nolan K (2004a). Catecholamines and aggression: the role of COMT and MAO polymorphisms. Ann N Y Acad Sci 1036: 393–398.
Volavka J, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome L et al (2004b). Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol 24: 225–228.
Volavka J, Kennedy JL, Ni X, Czobor P, Nolan K, Sheitman B et al (2004c). COMT158 polymorphism and hostility. Am J Med Genet B Neuropsychiatr Genet 127B: 28–29.
Volkow ND, Tancredi LR, Grant C, Gillespie H, Valentine A, Mullani N et al (1995). Brain glucose metabolism in violent psychiatric patients: a preliminary study. Psychiatry Res 61: 243–253.
Volman I, Verhagen L, den Ouden HE, Fernandez G, Rijpkema M, Franke B et al (2013). Reduced serotonin transporter availability decreases prefrontal control of the amygdala. J Neurosci 33: 8974–8979.
Wals M, Verhulst F (2005). Child and adolescent antecedents of adult mood disorders. Curr Opin Psychiatry 18: 15–19.
Wasman M, Flynn JP (1962). Directed attack elicited from hypothalamus. Arch Neurol 6: 220–227.
Watt MJ, Burke AR, Renner KJ, Forster GL (2009). Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci 123: 564–576.
Weaver KJ, Paul IA, Lin RC, Simpson KL (2010). Neonatal exposure to citalopram selectively alters the expression of the serotonin transporter in the hippocampus: dose-dependent effects. Anat Rec (Hoboken) 293: 1920–1932.
Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP et al (2006). Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313: 536–540.
Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R et al (2007). Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci 27: 684–691.
Willeit M, Praschak-Rieder N, Neumeister A, Pirker W, Asenbaum S, Vitouch O et al (2000). [123I]-beta-CIT SPECT imaging shows reduced brain serotonin transporter availability in drug-free depressed patients with seasonal affective disorder. Biol Psychiatry 47: 482–489.
Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW (2004). 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 176: 376–385.
Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K et al (2009). Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry 66: 1201–1209.
Yoon Y, McKenna MC, Rollins DA, Song M, Nuriel T, Gross SS et al (2013). Anxiety-associated alternative polyadenylation of the serotonin transporter mRNA confers translational regulation by hnRNPK. Proc Natl Acad Sci USA 110: 11624–11629.
Yu Q, Teixeira CM, Mahadevia D, Huang Y, Balsam D, Mann JJ et al (2014). Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol Psychiatry 19: 688–698.
Zalsman G, Huang YY, Harkavy-Friedman JM, Oquendo MA, Ellis SP, Mann JJ (2005). Relationship of MAO-A promoter (u-VNTR) and COMT (V158M) gene polymorphisms to CSF monoamine metabolites levels in a psychiatric sample of caucasians: A preliminary report. Am J Med Genet B Neuropsychiatr Genet 132B: 100–103.
Zalsman G, Huang YY, Oquendo MA, Burke AK, Hu XZ, Brent DA et al (2006). Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry 163: 1588–1593.
Zhang ZW (2003). Serotonin induces tonic firing in layer V pyramidal neurons of rat prefrontal cortex during postnatal development. J Neurosci 23: 3373–3384.
Zhuang X, Gross C, Santarelli L, Compan V, Trillat AC, Hen R (1999). Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharmacology 21 (2 Suppl): 52S–60S.
Zill P, Buttner A, Eisenmenger W, Moller HJ, Bondy B, Ackenheil M (2004). Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene in suicide victims. Biol Psychiatry 56: 581–586.
Acknowledgements
This work was supported by the Sackler Institute for Developmental Psychobiology and the National Institute of Mental Health (R01 MH099118; R00MH083044; P50MH90966).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suri, D., Teixeira, C., Cagliostro, M. et al. Monoamine-Sensitive Developmental Periods Impacting Adult Emotional and Cognitive Behaviors. Neuropsychopharmacol 40, 88–112 (2015). https://doi.org/10.1038/npp.2014.231
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.231
This article is cited by
-
Dopamine transporter blockade during adolescence increases adult dopamine function, impulsivity, and aggression
Molecular Psychiatry (2023)
-
Nine-month-long Social Isolation Changes the Levels of Monoamines in the Brain Structures of Rats: A Comparative Study of Neurochemistry and Behavior
Neurochemical Research (2023)
-
Early life adversity shapes neural circuit function during sensitive postnatal developmental periods
Translational Psychiatry (2022)
-
Early Life Social Stress Causes Sex- and Region-Dependent Dopaminergic Changes that Are Prevented by Minocycline
Molecular Neurobiology (2022)
-
Neuromodulatory effect of interleukin 1β in the dorsal raphe nucleus on individual differences in aggression
Molecular Psychiatry (2022)