Abstract
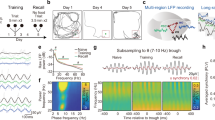
In humans, distinct processes within the hippocampus and rhinal cortex support declarative memory formation. But do these medial temporal lobe (MTL) substructures directly cooperate in encoding new memories? Phase synchronization of gamma-band electroencephalogram (EEG) oscillations (around 40 Hz) is a general mechanism of transiently connecting neural assemblies. We recorded depth-EEG from within the MTL of epilepsy patients performing a memorization task. Successful as opposed to unsuccessful memory formation was accompanied by an initial elevation of rhinal–hippocampal gamma synchronization followed by a later desynchronization, suggesting that effective declarative memory formation is accompanied by a direct and temporarily limited cooperation between both MTL substructures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Scoville, W. B. & Millner, B. J. Loss of recent memory after bilateral hippocampal lesions. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957).
Eichenbaum, H. A cortical-hippocampal system for declarative memory. Nat. Rev. Neurosci. 1, 41–50 (2000).
Gabrieli, J. D. E. Cognitive neuroscience of human memory. Annu. Rev. Neurosci. 49, 87–115 (1998).
Zola-Morgan, S. & Squire, L. R. Neuroanatomy of memory. Annu. Rev. Neurosci. 16, 547–563 (1993).
Otten, L. J., Henson, R. N. A. & Rugg, M. D. Depth of processing effects on neural correlates of memory encoding. Brain 124, 399–412 (2001).
Kirchhoff, B. A., Wagner, A. D., Maril, A. & Stern, C. E. Prefrontal-temporal circuitry for episodic and subsequent memory. J. Neurosci. 20, 6173–6180 (2000).
Henke, K., Weber, B., Kneifel, S., Wieser, H. G. & Buck, A. Human hippocampus associates information in memory. Proc. Natl. Acad. Sci. USA 96, 5884–5889 (1999).
Fernández, G., Brewer, J. B., Zhao, Z., Glover, G. H. & Gabrieli, J. D. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: a functional magnetic resonance imaging study with high acquisition rate. Hippocampus 9, 35–44 (1999).
Brewer, J. B., Zhao, Z., Desmond, J. E., Glover, G. H. & Gabrieli, J. D. E. Making memories: brain activity that predicts how well visual experience will be remembered. Science 281, 1185–1187 (1998).
Wagner, A. D. et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 281, 1188–1191 (1998).
Fernández, G. et al. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science 285, 1582–1585 (1999).
Lavenex, P. & Amaral, D. G. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus 10, 420–430 (2000).
Amaral, D. G. & Insausti, R. in The Human Nervous System (ed. Paxinos, G.) 711–755 (Academic, San Diego, 1990).
Witter, M. P., Groenewegen, H. J., Lopes da Silva, F. H. & Lohman, A. H. M. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog. Neurobiol. 33, 161–253 (1989).
Brown, M. W. & Aggleton, J. P. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2, 51–61 (2001).
Engel, A. K. & Singer, W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn. Sci. 5, 16–25 (2001).
Varela, F. J., Lachaux, J.-P., Rodriguez, E. & Martinerie, J. The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239 (2000).
Sauvé, K. Gamma-band synchronous oscillations: recent evidence regarding their functional significance. Conscious. Cogn. 8, 213–224 (1999).
Tallon-Baudry, C. & Bertrand, O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 3, 151–162 (1999).
Singer, W. Striving for coherence. Nature 397, 391–393 (1999).
Rodriguez, E. et al. Perception's shadow: long-distance synchronization of human brain activity. Nature 397, 430–433 (1999).
Miltner, W. H., Braun, C., Arnold, M., Witte, H. & Taub, E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature 397, 434–436 (1999).
Van Roost, D., Solymosi, L., Schramm, J., Van Oosterwyck, B. & Elger, C. E. Depth electrode implantation in the length axis of the hippocampus for the presurgical evaluation of medial temporal lobe epilepsy: a computed tomography-based stereotactic insertion technique and its accuracy. Neurosurgery 43, 819–826 (1998).
Morris, H. H. & Luders, H. Electrodes. Electroencephalogr. Clin. Neurophysiol. Suppl. 37, 3–26 (1985).
Paller, K. A., McCarthy, G., Roessler, E., Allison, T. & Wood, C. C. Potentials evoked in human and monkey medial temporal lobe during auditory and visual oddball paradigms. Electroencephalogr. Clin. Neurophysiol. 84, 269–279 (1992).
Hermann, B. P., Seidenberg, M., Schoenfeld, J. & Davies, K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch. Neurol. 54, 369–376 (1997).
Fernández, G. et al. Event-related potentials of verbal encoding into episodic memory: dissociation between the effects of subsequent memory performance and distinctiveness. Psychophysiology 35, 709–720 (1998).
Elger, C. E. et al. Human temporal lobe potentials in verbal learning and memory processes. Neuropsychologia 35, 657–667 (1997).
Hirai, N., Uchida, S., Maehara, T., Okubo, Y. & Shimizu, H. Enhanced gamma (30–150 Hz) frequency in the human medial temporal lobe. Neuroscience 90, 1149–1155 (1999).
Menon, V. et al. Spatio-temporal correlations in human gamma band electrocorticograms. Electroencephalogr. Clin. Neurophysiol. 98, 89–102 (1996).
Bullock, T. H. et al. EEG coherence has structure in the millimetre domain: subdural and hippocampal recordings from epileptic patients. Electroencephalogr. Clin. Neurophysiol. 95, 161–177 (1995).
McCarthy, G., Nobre, A. C., Bentin, S. & Spencer, D. D. Language-related field potentials in the anterior-medial temporal lobe: I. Intracranial distribution and neural generators. J. Neurosci. 15, 1080–1089 (1995).
Klee, M., & Rall, W. Computed potentials of cortically arranged populations of neurons. J. Neurophysiol. 40, 647–666 (1977).
Traub, R. D., Whittington, M. A., Stanford, I. M. & Jefferys, J. G. R. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 383, 621–624 (1996).
Kreiman, G., Koch, C. & Fried, I. Imagery neurons in the human brain. Nature 408, 357–361 (2000).
Steinmetz, P. N. et al. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404, 187–190 (2000).
Desmedt, J. E. & Tomberg, C. Transient phase-locking of 40 Hz electrical oscillations in prefrontal and parietal human cortex reflects the process of conscious somatic perception. Neurosci. Lett. 168, 126–129 (1994).
Müller, M. M., Gruber, T. & Keil, A. Modulation of induced gamma band activity in the human EEG by attention and visual information processing. Int. J. Psychophysiol. 38, 283–299 (2000).
LaBerge, D. Attention, awareness, and the triangular circuit. Conscious. Cogn. 6, 149–181 (1997).
Traub, R. D., Whittington, M. A., Buhl, E. H., Jefferys, J. G. & Faulkner, H. J. On the mechanism of the γ_β frequency shift in neuronal oscillations induced in rat hippocampal slices by tetanic stimulation. J. Neurosci. 19, 1088–1105 (1999).
Whittington, M. A., Traub, R. D., Faulkner, H. J., Stanford, I. M. & Jefferys, J. G. Recurrent excitatory postsynaptic potentials induced by synchronized fast cortical oscillations. Proc. Natl. Acad. Sci. USA 94, 12198–12203 (1997).
Bliss, T. V. P. & Lomo, T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. (Lond.) 232, 331–356 (1973).
Craik, F. I. M., Govoni, R., Naveh-Benjamin, M. & Anderson, N. D. The effects of divided attention on encoding and retrieval processes in human memory. J. Exp. Psychol. Gen. 125, 159–180 (1996).
Buzsaki, G. The hippocampo-neocortical dialogue. Cereb. Cortex 6, 81–92 (1996).
Baayen, R. H., Piepenbrock, R. & van Rijn, H. CELEX Lexical Database (Linguistic Data Consortium, University of Pennsylvania, Philadelphia, 1993).
Jackson, G. D. & Duncan, J. S. MRI Neuroanatomy (Churchill Livingstone, New York, 1996).
Daubechies, I. The wavelet transform, time-frequency localisation and signal analysis. IEEE Trans. Inform. Theory 36, 961–1005 (1990).
Mardia, K. V. Probability and Mathematical Statistics: Statistics of Directional Data (Academic, London, 1972).
Huynh, H. & Feldt, L. S. Estimation of the box correction for degrees of freedom from sample data in the randomized plot and split plot designs. J. Educ. Stat. 1, 69–82 (1976).
Acknowledgements
This research was supported by the Deutsche Forschungsgemeinschaft (DFG Fe479/4-1). We wish to thank H. Beck, W. Burr, A. Engel, C. Koch, M. Reuber and I. Tendolkar for comments on earlier drafts of the manuscript. We also thank H. Urbach for providing the MR images and I. Blümcke for providing the histopathological reports.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fell, J., Klaver, P., Lehnertz, K. et al. Human memory formation is accompanied by rhinal–hippocampal coupling and decoupling. Nat Neurosci 4, 1259–1264 (2001). https://doi.org/10.1038/nn759
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn759
This article is cited by
-
Enhancing memory capacity by experimentally slowing theta frequency oscillations using combined EEG-tACS
Scientific Reports (2022)
-
Priority coding in the visual system
Nature Reviews Neuroscience (2022)
-
Developmental decrease of entorhinal-hippocampal communication in immune-challenged DISC1 knockdown mice
Nature Communications (2021)
-
EEG theta responses induced by emoji semantic violations
Scientific Reports (2021)
-
Moment-by-moment tracking of naturalistic learning and its underlying hippocampo-cortical interactions
Nature Communications (2021)