Abstract
The serotonergic raphe nuclei are involved in regulating brain states over timescales of minutes and hours. We examined more rapid effects of raphe activation on two classes of principal neurons in the mouse olfactory bulb, mitral and tufted cells, which send olfactory information to distinct targets. Brief stimulation of the raphe nuclei led to excitation of tufted cells at rest and potentiation of their odor responses. While mitral cells at rest were also excited by raphe activation, their odor responses were bidirectionally modulated, leading to improved pattern separation of odors. In vitro whole-cell recordings revealed that specific optogenetic activation of raphe axons affected bulbar neurons through dual release of serotonin and glutamate. Therefore, the raphe nuclei, in addition to their role in neuromodulation of brain states, are also involved in fast, sub-second top-down modulation similar to cortical feedback. This modulation can selectively and differentially sensitize or decorrelate distinct output channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lee, S.-H. & Dan, Y. Neuromodulation of brain states. Neuron 76, 209–222 (2012).
Marder, E. Neuromodulation of neuronal circuits: back to the future. Neuron 76, 1–11 (2012).
Cools, R., Roberts, A.C. & Robbins, T.W. Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn. Sci. 12, 31–40 (2008).
Dayan, P. & Huys, Q.J.M. Serotonin in affective control. Annu. Rev. Neurosci. 32, 95–126 (2009).
Liu, Z. et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81, 1360–1374 (2014).
Dacks, A.M., Green, D.S., Root, C.M., Nighorn, A.J. & Wang, J.W. Serotonin modulates olfactory processing in the antennal lobe of Drosophila. J. Neurogenet. 23, 366–377 (2009).
Fonseca, M.S., Murakami, M. & Mainen, Z.F. Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing. Curr. Biol. 25, 306–315 (2015).
Hurley, L.M., Devilbiss, D.M. & Waterhouse, B.D. A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr. Opin. Neurobiol. 14, 488–495 (2004).
Petzold, G.C., Hagiwara, A. & Murthy, V.N. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat. Neurosci. 12, 784–791 (2009).
Hilaire, G. et al. The role of serotonin in respiratory function and dysfunction. Respir. Physiol. Neurobiol. 174, 76–88 (2010).
Jacobs, B.L. & Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229 (1992).
Ray, R.S. et al. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333, 637–642 (2011).
Hannon, J. & Hoyer, D. Molecular biology of 5-HT receptors. Behav. Brain Res. 195, 198–213 (2008).
Cohen, J.Y., Amoroso, M.W. & Uchida, N. Serotonergic neurons signal reward and punishment on multiple timescales. Elife 4, e06346 (2015).
Ranade, S.P. & Mainen, Z.F. Transient firing of dorsal raphe neurons encodes diverse and specific sensory, motor, and reward events. J. Neurophysiol. 102, 3026–3037 (2009).
Rhoades, R.W., Bennett-Clarke, C.A., Shi, M.Y. & Mooney, R.D. Effects of 5-HT on thalamocortical synaptic transmission in the developing rat. J. Neurophysiol. 72, 2438–2450 (1994).
Suzuki, Y., Kiyokage, E., Sohn, J., Hioki, H. & Toida, K. Structural basis for serotonergic regulation of neural circuits in the mouse olfactory bulb. J. Comp. Neurol. 523, 262–280 (2015).
Varga, V. et al. Fast synaptic subcortical control of hippocampal circuits. Science 326, 449–453 (2009).
Nagayama, S., Homma, R. & Imamura, F. Neuronal organization of olfactory bulb circuits. Front. Neural Circuits 8, 98 (2014).
Haberly, L.B. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem. Senses 26, 551–576 (2001).
Fukunaga, I., Berning, M., Kollo, M., Schmaltz, A. & Schaefer, A.T. Two distinct channels of olfactory bulb output. Neuron 75, 320–329 (2012).
Giessel, A.J. & Datta, S.R. Olfactory maps, circuits and computations. Curr. Opin. Neurobiol. 24, 120–132 (2014).
McLean, J.H. & Shipley, M.T. Serotonergic afferents to the rat olfactory bulb: I. Origins and laminar specificity of serotonergic inputs in the adult rat. J. Neurosci. 7, 3016–3028 (1987).
Steinfeld, R., Herb, J.T., Sprengel, R., Schaefer, A.T. & Fukunaga, I. Divergent innervation of the olfactory bulb by distinct raphe nuclei. J. Comp. Neurol. 523, 805–813 (2015).
Liu, S., Aungst, J.L., Puche, A.C. & Shipley, M.T. Serotonin modulates the population activity profile of olfactory bulb external tufted cells. J. Neurophysiol. 107, 473–483 (2012).
Hardy, A., Palouzier-Paulignan, B., Duchamp, A., Royet, J.-P. & Duchamp-Viret, P. 5-Hydroxytryptamine action in the rat olfactory bulb: in vitro electrophysiological patch-clamp recordings of juxtaglomerular and mitral cells. Neuroscience 131, 717–731 (2005).
Nakamura, K., Matsumoto, M. & Hikosaka, O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J. Neurosci. 28, 5331–5343 (2008).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Haddad, R. et al. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat. Neurosci. 16, 949–957 (2013).
Wachowiak, M. All in a sniff: olfaction as a model for active sensing. Neuron 71, 962–973 (2011).
Adam, Y. et al. Functional transformations of odor inputs in the mouse olfactory bulb. Front. Neural Circuits 8, 129 (2014).
Kikuta, S., Fletcher, M.L., Homma, R., Yamasoba, T. & Nagayama, S. Odorant response properties of individual neurons in an olfactory glomerular module. Neuron 77, 1122–1135 (2013).
Zhao, S. et al. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat. Methods 8, 745–752 (2011).
Kapoor, V. & Urban, N.N. Glomerulus-specific, long-latency activity in the olfactory bulb granule cell network. J. Neurosci. 26, 11709–11719 (2006).
Gire, D.H. et al. Mitral cells in the olfactory bulb are mainly excited through a multistep signaling path. J. Neurosci. 32, 2964–2975 (2012).
Najac, M., De Saint Jan, D., Reguero, L., Grandes, P. & Charpak, S. Monosynaptic and polysynaptic feed-forward inputs to mitral cells from olfactory sensory neurons. J. Neurosci. 31, 8722–8729 (2011).
Friedrich, R.W. & Wiechert, M.T. Neuronal circuits and computations: pattern decorrelation in the olfactory bulb. FEBS Lett. 588, 2504–2513 (2014).
Miyazaki, K.W. et al. Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr. Biol. 24, 2033–2040 (2014).
Gracia-Llanes, F.J. et al. Synaptic connectivity of serotonergic axons in the olfactory glomeruli of the rat olfactory bulb. Neuroscience 169, 770–780 (2010).
Commons, K.G. Locally collateralizing glutamate neurons in the dorsal raphe nucleus responsive to substance P contain vesicular glutamate transporter 3 (VGLUT3). J. Chem. Neuroanat. 38, 273–281 (2009).
Fremeau, R.T. Jr., Voglmaier, S., Seal, R.P. & Edwards, R.H. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 27, 98–103 (2004).
Qi, J. et al. A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat. Commun. 5, 5390 (2014).
Okaty, B.W. et al. Multi-scale molecular deconstruction of the serotonin neuron system. Neuron. 88, 774–791 (2015).
Otazu, G.H., Chae, H., Davis, M.B. & Albeanu, D.F. Cortical feedback decorrelates olfactory bulb output in awake mice. Neuron 86, 1461–1477 (2015).
Burton, S.D. & Urban, N.N. Greater excitability and firing irregularity of tufted cells underlies distinct afferent-evoked activity of olfactory bulb mitral and tufted cells. J. Physiol. (Lond.) 592, 2097–2118 (2014).
Scott, J.W. Electrophysiological identification of mitral and tufted cells and distributions of their axons in olfactory system of the rat. J. Neurophysiol. 46, 918–931 (1981).
Kiselycznyk, C.L., Zhang, S. & Linster, C. Role of centrifugal projections to the olfactory bulb in olfactory processing. Learn. Mem. 13, 575–579 (2006).
Rothermel, M., Carey, R.M., Puche, A., Shipley, M.T. & Wachowiak, M. Cholinergic inputs from Basal forebrain add an excitatory bias to odor coding in the olfactory bulb. J. Neurosci. 34, 4654–4664 (2014).
Shea, S.D., Katz, L.C. & Mooney, R. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J. Neurosci. 28, 10711–10719 (2008).
Atasoy, D., Aponte, Y., Su, H.H. & Sternson, S.M. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J. Neurosci. 28, 7025–7030 (2008).
Soucy, E.R., Albeanu, D.F., Fantana, A.L., Murthy, V.N. & Meister, M. Precision and diversity in an odor map on the olfactory bulb. Nat. Neurosci. 12, 210–220 (2009).
Markopoulos, F., Rokni, D., Gire, D.H. & Murthy, V.N. Functional properties of cortical feedback projections to the olfactory bulb. Neuron 76, 1175–1188 (2012).
Acknowledgements
We thank the members of Murthy and Dulac labs for discussions throughout the project, and D. Gire, J. Cohen and M. Thanawala for comments on the manuscript. This work was supported by research grants (DC011291 and DC014453) from the US National Institutes of Health (V.N.M.), a seed grant from the Harvard Brain Initiative (V.N.M.) and fellowships from the US National Science Foundation and the Mortimer and Theresa Sackler Foundation (A.C.P.).
Author information
Authors and Affiliations
Contributions
A.C.P., V.K. and V.N.M. designed the project. A.C.P. and V.K. performed in vivo experiments; A.C.P. and P.A. performed and analyzed histological experiments; V.K. perform in vitro experiments. A.C.P. and V.K. analyzed the data with V.N.M.'s guidance. V.N.M. supervised the entire project. A.C.P., V.K. and V.N.M. wrote the paper with input from P.A.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
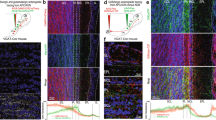
Supplementary Figure 1 Neuronal populations in the Tbx21-Cre (Tbet-Cre) mouse and 5-HT receptors in the OB
a) Tbet Cre mouse olfactory bulb injected with AAV 2.9 flex Gcamp6s virus showing sparse labelling of principal neurons of the OB.
b) 5-HT receptor 2a antibody (green, 1/1000 dilution, abcam ab66049) stains heavily in the external plexiform layer (EPL) and on MC bodies. Labeling performed in Tbet-Cre x Flex tdTomato mouse, so all principal cells are red.
c) Inhibitory 5-HT receptor 1a (red, 1/1000 dilution, abcam ab101914) stains non-GABAergic cells both in the EPL and MC layer (c1) and the glomerular layer (GL, c2). Staining performed in the GAD67-GFP mouse, so inhibitory cells are green.
d) Inhibitory 5-HT receptor 1b (red, 1/1000 dilution, abcam ab102700) stains non-GABAergic cells in the OB. Staining performed in the GAD67-GFP mouse (green). Granule Cell Layer (GCL).
Supplementary Figure 3 Sensitization of TC odor responses by raphe stimulation
a) Scatter plot showing sensitization in TC odor responses in 6 animals. Y axis is the observed response to paired odor and raphe stimulation minus raphe only stimulation response and x axis is odor only response.
b) Bar plot showing mean change in TC responses across animals. Mean response is calculated as the difference between paired odor plus raphe responses and (odor only + raphe only) responses.
c) Scatter plot showing the predicted response (linear model, R2 = 0.7611) vs observed response for 6 animals (1087 odor cell pairs).
d) Scatter plot showing the predicted response (linear model, R2 = 0.8638) vs observed response (1087 odor cell pairs).
Supplementary Figure 4 Odor inputs to the OB are not affected by brief raphe stimulation
a) Resting fluorescence image of glomeruli from an OMP-GCaMP3 mouse.
b) Example traces of odor responses from a single OMP-GCaMP3 glomerulus. Odor duration denoted by green bar at bottom.
c) Odor responses of olfactory sensory neurons measured from multiple glomeruli (left), in an OMP-GCaMP3 mouse, were not altered when the same odor was paired with raphe activation (right).
d) Scatter plot of all glomeruli odor pairs comparing average odor responses (dF/F) with and without raphe activation. Dashes are the unity line (p>0.3, Wilcoxon signed-rank, 2 mice, 26 glomeruli).
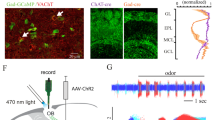
Supplementary Figure 5 Raphe projections to the main olfactory bulb
a) Anti-GFP antibody (green, 1/500 dilution; Aves GFP-1010) and anti-serotonin antibody (red, 1/1000 dilution; Sigma S5545) in a TPH2-ChR2 animal stains heavily in the glomerular layer and granule cell layer.
b) Zoomed in version of images shown in a, depicting the heterogeneity of raphe projections in different layers of the olfactory bulb.
c) Bar plots summarizing the density of raphe projections in the olfactory bulb as the mean pixel intensity in the different layers of the olfactory bulb (glomerular layer: 1.0, external plexiform layer: 0.26, mitral cell layer: 0.35, granule cell layer: 0.48, n=4). Data normalized to glomerular layer.
Supplementary Figure 6 Modulation of odor-evoked activity of principal neurons in the olfactory bulb by raphe stimulation in an awake animal
a) Time course of fluorescence changes in 36 TCs in an awake animal in response to ethyl valerate with (right) and without (left) raphe stimulation.
b) Similar plot showing time course responses of 23 MCs in an awake animal in response to ethyl valerate with (right) and without (left) raphe stimulation.
c) Scatter plot showing sensitization of odor responses in TCs with raphe stimulation in awake animals (468 cell-odor pairs, n=2). Mean change in odor response 23.71 ± 1 % (median change of 19.79 %).
d) Scatter plot showing bidirectional modulation of odor responses with raphe stimulation in awake animals (282 cell-odor pairs, n=2). Mean change in odor response 1.94 ± 0.9 % (median change of 1.79 %).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 1119 kb)
A 3-dimensional stack showing images of tufted and mitral cells.
Stack is made up of 1 micron optical sections through 360 microns depth, and shows cells imaged in a living mouse with a multiphoton microscope. Green colour represents GCaMP6s fluorescence. Movie starts with the most superficial section with apical tufts visible. Tufted cell somata are visible a bit deeper and past the external plexiform layer (where many lateral dendrites are visible) mitral cell somata become visible. (AVI 6436 kb)
Rights and permissions
About this article
Cite this article
Kapoor, V., Provost, A., Agarwal, P. et al. Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels. Nat Neurosci 19, 271–282 (2016). https://doi.org/10.1038/nn.4219
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4219
This article is cited by
-
A preoptic neuronal population controls fever and appetite during sickness
Nature (2022)
-
Extrinsic neuromodulation in the rodent olfactory bulb
Cell and Tissue Research (2021)
-
Pro-neurogenic effect of fluoxetine in the olfactory bulb is concomitant to improvements in social memory and depressive-like behavior of socially isolated mice
Translational Psychiatry (2020)
-
Coronaviruses: a challenge of today and a call for extended human postmortem brain analyses
Journal of Neural Transmission (2020)
-
Target specific functions of EPL interneurons in olfactory circuits
Nature Communications (2019)