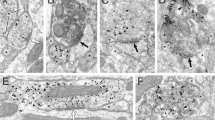
Abstract
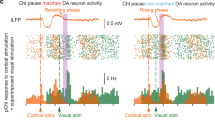
Dopamine (DA) is required for hippocampal-dependent memory and long-term potentiation (LTP) at CA1 Schaffer collateral (SC) synapses. It is therefore surprising that exogenously applied DA has little effect on SC synapses, but suppresses CA1 perforant path (PP) inputs. To examine DA actions under more physiological conditions, we used optogenetics to release DA from ventral tegmental area inputs to hippocampus. Unlike exogenous DA application, optogenetic release of DA caused a bidirectional, activity-dependent modulation of SC synapses, with no effect on PP inputs. Low levels of DA release, simulating tonic DA neuron firing, depressed the SC response through a D4 receptor–dependent enhancement of feedforward inhibition mediated by parvalbumin-expressing interneurons. Higher levels of DA release, simulating phasic firing, increased SC responses through a D1 receptor–dependent enhancement of excitatory transmission. Thus, tonic-phasic transitions in DA neuron firing in response to motivational demands may cause a modulatory switch from inhibition to enhancement of hippocampal information flow.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Faure, A., Haberland, U., Conde, F. & El Massioui, N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J. Neurosci. 25, 2771–2780 (2005).
Lisman, J.E. & Grace, A.A. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46, 703–713 (2005).
Bethus, I., Tse, D. & Morris, R.G. Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor–dependent paired associates. J. Neurosci. 30, 1610–1618 (2010).
Rossato, J.I., Bevilaqua, L.R., Izquierdo, I., Medina, J.H. & Cammarota, M. Dopamine controls persistence of long-term memory storage. Science. 325, 1017–1020 (2009).
Wilkerson, A. & Levin, E.D. Ventral hippocampal dopamine D1 and D2 systems and spatial working memory in rats. Neuroscience 89, 743–749 (1999).
McNamara, C.G., Tejero-Cantero, A., Trouche, S., Campo-Urriza, N. & Dupret, D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat. Neurosci. 17, 1658–1660 (2014).
Otmakhova, N.A. & Lisman, J.E. Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J. Neurosci. 19, 1437–1445 (1999).
Ito, H.T. & Schuman, E.M. Frequency-dependent signal transmission and modulation by neuromodulators. Front. Neurosci. 2, 138–144 (2008).
Huang, Y.Y. & Kandel, E.R. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc. Natl. Acad. Sci. USA 92, 2446–2450 (1995).
Swanson, L.W. The projections of the ventral tegmental area and adjacent regions—a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 9, 321–353 (1982).
Gasbarri, A., Packard, M.G., Campana, E. & Pacitti, C. Anterograde and retrograde tracing of projections from the ventral tegmental area to the hippocampal formation in the rat. Brain Res. Bull. 33, 445–452 (1994).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Sulzer, D. et al. Dopamine neurons make glutamatergic synapses in vitro. J. Neurosci. 18, 4588–4602 (1998).
Kwon, O.B. et al. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc. Natl. Acad. Sci. USA 105, 15587–15592 (2008).
Tritsch, N.X., Ding, J.B. & Sabatini, B.L. Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490, 262–266 (2012).
Defagot, M.C., Malchiodi, E.L., Villar, M.J. & Antonelli, M.C. Distribution of D4 dopamine receptor in rat brain with sequence-specific antibodies. Brain Res. Mol. Brain Res. 45, 1–12 (1997).
Buzsáki, G. Feedforward Inhibition in the hippocampal formation. Prog. Neurobiol. 22, 131–153 (1984).
Hu, H., Gan, J. & Jonas, P. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science 345, 1255263 (2014).
Mrzljak, L. et al. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 381, 245–248 (1996).
Andersson, R.H. et al. Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proc. Natl. Acad. Sci. USA 109, 13118–13123 (2012).
Magnus, C.J. et al. Chemical and genetic engineering of selective ion channel-ligand interactions. Science 333, 1292–1296 (2011).
Basu, J. et al. A cortico-hippocampal learning rule shapes inhibitory microcircuit activity to enhance hippocampal information flow. Neuron 79, 1208–1221 (2013).
Hippenmeyer, S. et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 3, e159 (2005).
Armstrong, C. & Soltesz, I. Basket cell dichotomy in microcircuit function. J. Physiol. (Lond.) 590, 683–694 (2012).
Nissen, W., Szabo, A., Somogyi, J., Somogyi, P. & Lamsa, K.P. Cell type–specific long-term plasticity at glutamatergic synapses onto hippocampal interneurons expressing either parvalbumin or CB1 cannabinoid receptor. J. Neurosci. 30, 1337–1347 (2010).
Yuen, E.Y., Zhong, P. & Yan, Z. Homeostatic regulation of glutamatergic transmission by dopamine D4 receptors. Proc. Natl. Acad. Sci. USA 107, 22308–22313 (2010).
Gasbarri, A., Sulli, A., Innocenzi, R., Pacitti, C. & Brioni, J.D. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience 74, 1037–1044 (1996).
Wang, L.P. et al. NMDA receptors in dopaminergic neurons are crucial for habit learning. Neuron 72, 1055–1066 (2011).
Fuxe, K. et al. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 90, 82–100 (2010).
Smith, C.C. & Greene, R.W. CNS dopamine transmission mediated by noradrenergic innervation. J. Neurosci. 32, 6072–6080 (2012).
Hsu, K.S. Characterization of dopamine receptors mediating inhibition of excitatory synaptic transmission in the rat hippocampal slice. J. Neurophysiol. 76, 1887–1895 (1996).
Gainetdinov, R.R., Premont, R.T., Bohn, L.M., Lefkowitz, R.J. & Caron, M.G. Desensitization of G protein–coupled receptors and neuronal functions. Annu. Rev. Neurosci. 27, 107–144 (2004).
Tritsch, N.X. & Sabatini, B.L. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50 (2012).
Bolton, M.M., Pittman, A.J. & Lo, D.C. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J. Neurosci. 20, 3221–3232 (2000).
Zakharenko, S.S. et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1–CA3 synapses. Neuron 39, 975–990 (2003).
Cobb, S.R., Buhl, E.H., Halasy, K., Paulsen, O. & Somogyi, P. Synchronization of neuronal-activity in hippocampus by individual GABAergic interneurons. Nature 378, 75–78 (1995).
Pouille, F. & Scanziani, M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163 (2001).
Royer, S. et al. Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci. 15, 769–775 (2012).
Losonczy, A., Zemelman, B.V., Vaziri, A. & Magee, J.C. Network mechanisms of theta related neuronal activity in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 13, 967–972 (2010).
Cardin, J.A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Murray, A.J. et al. Parvalbumin-positive CA1 interneurons are required for spatial working but not for reference memory. Nat. Neurosci. 14, 297–299 (2011).
Glickfeld, L.L., Atallah, B.V. & Scanziani, M. Complementary modulation of somatic inhibition by opioids and cannabinoids. J. Neurosci. 28, 1824–1832 (2008).
Lapray, D. et al. Behavior-dependent specialization of identified hippocampal interneurons. Nat. Neurosci. 15, 1265–1271 (2012).
Lisman, J.E. & Otmakhova, N.A. Storage, recall and novelty detection of sequences by the hippocampus: Elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus 11, 551–568 (2001).
Schultz, W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 30, 259–288 (2007).
Grace, A.A., Floresco, S.B., Goto, Y. & Lodge, D.J. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30, 220–227 (2007).
Kawaguchi, Y. & Hama, K. Fast-spiking nonpyramidal cells in the hippocampal CA3 region, dentate gyrus and subiculum of rats. Brain Res. 425, 351–355 (1987).
Acknowledgements
We thank J. Basu (New York University) for her theoretical and technical support with experiments and for helpful comments on the manuscript. We are grateful to S. Sternson for providing the PSEM compound and PSAM construct (Janelia Research Campus, Howard Hughes Medical Institute). The work was supported by funding from the Howard Hughes Medical Institute and a grant from the US National Institutes of Health (5T32MH015174).
Author information
Authors and Affiliations
Contributions
Z.B.R. conducted the electrophysiological experiments. Z.B.R. and S.C. performed the immunohistochemistry. Z.B.R. and S.A.S. designed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 DA release does not induce rapid changes in intrinsic membrane properties
(a) Schematic of the light burst, which consisted of 3 pulses (5 ms each) with a 10 ms interpulse interval (66.7 Hz). (b) Input resistance before and after photostimulation. Pre = five minutes prior to the light burst protocol. Post = 15 minutes following a single light burst. (c) Input resistance before and after 20 ΩM DA bath application.
Supplementary Figure 2 IPSCs evoked by direct stimulation of GABAergic interneurons throughout CA1 are unaffected by DA release.
(a) Effects of DA release on direct inhibition of CA1 PNs as measured by the IPSC recorded under whole cell voltage clamp at + 10 mV in the presence of NBQX and D-APV. (b) Comparison of the population mean direct IPSCs recorded from CA1 PNs in response to electrical stimulation using an electrode placed in the indicated four layers of CA1, plus the feedforward IPSC evoked by SC stimulation with excitation intact. For direct inhibition experiments, NBQX and D-AP5 were included in the bath solution. Photostimulation of DA release caused no statistically significant change in the direct IPSCs evoked with stimulating electrode in SO. In contrast DA release significantly increased the feedforward IPSC (P values?). (c) Current recording in CA1 pyramidal neurons at a holding potential of +10 mV. Photostimulation protocol is equivalent to the one used in the previous current clamp experiments and shown to scale.
Supplementary Figure 3 Prolonged optogenetic stimulation has no effect on feedforward IPSC.
(a) Experimental configuration. (b) Effect of prolonged optogenetic stimulation on the SC-evoked IPSC measured in voltage clamp at +10 mV as in Figure 3. Closed blue circles: effect of single burst stimulation on the mean IPSC peak amplitude normalized to the baseline. Closed black circles: effect of prolonged burst stimulation.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 779 kb)
Rights and permissions
About this article
Cite this article
Rosen, Z., Cheung, S. & Siegelbaum, S. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci 18, 1763–1771 (2015). https://doi.org/10.1038/nn.4152
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.4152
This article is cited by
-
Non-canonical interplay between glutamatergic NMDA and dopamine receptors shapes synaptogenesis
Nature Communications (2024)
-
Dopamine neuron degeneration in the Ventral Tegmental Area causes hippocampal hyperexcitability in experimental Alzheimer’s Disease
Molecular Psychiatry (2024)
-
Purkinje cell dopaminergic inputs to astrocytes regulate cerebellar-dependent behavior
Nature Communications (2023)
-
The effect of prediction error on episodic memory encoding is modulated by the outcome of the predictions
npj Science of Learning (2023)
-
Upregulation of Ca2+-binding proteins contributes to VTA dopamine neuron survival in the early phases of Alzheimer’s disease in Tg2576 mice
Molecular Neurodegeneration (2022)