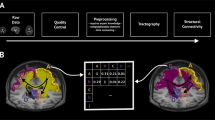
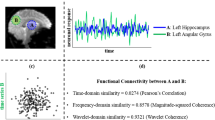
Abstract
Intrinsic functional connectivity magnetic resonance imaging (fcMRI) has emerged as a powerful tool for mapping large-scale networks in the human brain. Robust and reliable functionally coupled networks can be detected in individuals that echo many known features of anatomical organization. Features of brain organization have been discovered, including descriptions of distributed large-scale networks interwoven throughout association cortex, interactions (including anticorrelations) between brain networks and insights into the topography of subcortical structures. But interpreting fcMRI is complicated by several factors. Functional coupling changes dynamically, suggesting that it is constrained by, but not fully dictated by, anatomic connectivity. Critically to study of between-group differences, fcMRI is sensitive to head motion and to differences in the mental states of participants during the scans. We discuss the potential of fcMRI in the context of its limitations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ogawa, S. et al. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging: a comparison of signal characteristics with a biophysical model. Biophys. J. 64, 803–812 (1993).
Biswal, B., Yetkin, F.Z., Haughton, V.M. & Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995).
Greicius, M.D., Krasnow, B., Reiss, A.L. & Menon, V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 100, 253–258 (2003).
Fox, M.D., Corbetta, M., Snyder, A.Z., Vincent, J.L. & Raichle, M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. USA 103, 10046–10051 (2006).
De Luca, M., Beckmann, C.F., De Stefano, N., Matthews, P.M. & Smith, S.M. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage 29, 1359–1367 (2006).
Damoiseaux, J.S. et al. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 103, 13848–13853 (2006).
Seeley, W.W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007).
Margulies, D.S. et al. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37, 579–588 (2007).
Dosenbach, N.U.F. et al. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. USA 104, 11073–11078 (2007).
Greicius, M. Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 21, 424–430 (2008).
Zhang, D. & Raichle, M.E. Disease and the brain's dark energy. Nat. Rev. Neurol. 6, 15–28 (2010).
Buckner, R.L., Krienen, F.M., Castellanos, A., Diaz, J.C. & Yeo, B.T.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 2322–2345 (2011).
Lu, J. et al. Focal pontine lesions provide evidence that intrinsic functional connectivity reflects polysynaptic anatomical pathways. J. Neurosci. 31, 15065–15071 (2011).
Johnston, J.M. et al. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J. Neurosci. 28, 6453–6458 (2008).
Damoiseaux, J.S. & Greicius, M.D. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 213, 525–533 (2009).
Greicius, M.D., Supekar, K., Menon, V. & Dougherty, R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex 19, 72–78 (2009).
Honey, C.J. et al. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. USA 106, 2035–2040 (2009).
Vincent, J.L. et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86 (2007).
Margulies, D.S. et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc. Natl. Acad. Sci. USA 106, 20069–20074 (2009).
Mars, R.B. et al. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J. Neurosci. 31, 4087–4100 (2011).
Hill, J. et al. Similar patterns of cortical expansion during human development and evolution. Proc. Natl. Acad. Sci. USA 107, 13135–13140 (2010).
Tyszka, J.M., Kennedy, D.P., Adolphs, R. & Paul, L.K. Intact bilateral resting-state networks in the absence of the corpus callosum. J. Neurosci. 31, 15154–15162 (2011).
Shirer, W.R., Ryali, S., Rykhlevskaia, E., Menon, V. & Greicius, M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb. Cortex 22, 158–165 (2012).
Lewis, C.M., Baldassarre, A., Committeri, G., Romani, G.L. & Corbetta, M. Learning sculpts the spontaneous activity of the resting human brain. Proc. Natl. Acad. Sci. USA 106, 17558–17563 (2009).
Hutchison, R.M., Womelsdorf, T., Gati, J.S., Everling, S. & Menon, R.S. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum. Brain Mapp. doi:dx10.1002/hbm.22058 (2012).
Handwerker, D.A., Gonzalez-Castillo, J., D'Esposito, M. & Bandettini, P.A. The continuing challenge of understanding and modeling hemodynamic variation in fMRI. Neuroimage 62, 1017–1023 (2012).
Van Dijk, K.R.A., Sabuncu, M.R. & Buckner, R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438 (2012).
Power, J.D., Barnes, K.A., Snyder, A.Z., Schlaggar, B.L. & Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Birn, R.M., Smith, M.A., Jones, T.B. & Bandettini, P.A. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage 40, 644–654 (2008).
Deco, G. & Corbetta, M. The dynamical balance of the brain at rest. Neuroscientist 17, 107–123 (2011).
Andrews-Hanna, J.R. The brain's default network and its adaptive role in internal mentation. Neuroscientist 18, 251–270 (2012).
Spreng, R.N., Mar, R.A. & Kim, A.S.N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 21, 489–510 (2009).
Smith, S.M. et al. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 106, 13040–13045 (2009).
Bleuler, E. Dementia Praecox or the Group of Schizophrenias (ed. Zinkin, J.) (International Universities Press; New York, 1950).
Nolen-Hoeksma, S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J. Abnorm. Psychol. 109, 504–511 (2000).
Cohen, A.L. et al. Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage 41, 45–57 (2008).
Yeo, B.T.T. et al. The organization of human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165 (2011).
Power, J.D. et al. Functional network organization of the human brain. Neuron 72, 665–678 (2011).
Kaas, J.H. The organization of neocortex in mammals: implications for theories of brain function. Annu. Rev. Psychol. 38, 129–151 (1987).
Wig, G.S., Schlaggar, B.L. & Petersen, S.E. Concepts and principles in the analysis of brain networks. Ann. NY Acad. Sci. 1224, 126–146 (2011).
Maunsell, J.H.R. & Van Essen, D.C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci. 3, 2563–2586 (1983).
Passingham, R.E., Stephan, K.E. & Kötter, R. The anatomical basis of functional localization in the cortex. Nat. Rev. Neurosci. 3, 606–616 (2002).
Rosa, M.G.P. & Tweedale, R. Brain maps, great and small: lessons from comparative studies of primate visual cortical organization. Phil. Trans. R. Soc. Lond. B. Biol. Sci. 360, 665–691 (2005).
Rakic, P. Specification of cerebral cortical areas. Science 241, 170–176 (1988).
Mueller, S. et al. Individual variability in functional connectivity architecture of the human brain. Neuron 77, 586–595 (2013).
Goldman-Rakic, P.S. Topography of cognition: parallel distributed networks in primate association cortex. Annu. Rev. Neurosci. 11, 137–156 (1988).
Manni, E. & Petrosini, L. A century of cerebellar somatotopy: a debated representation. Nat. Rev. Neurosci. 5, 241–249 (2004).
Strick, P.L., Dum, R.P. & Fiez, J.A. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 32, 413–434 (2009).
Habas, C. et al. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 29, 8586–8594 (2009).
O'Reilly, J.X., Beckmann, C.F., Tomassini, V., Ramnani, N. & Johansen-Berg, H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex 20, 953–965 (2010).
Mesulam, M. The evolving landscape of human cortical connectivity: facts and inferences. Neuroimage 62, 2182–2189 (2012).
Fransson, P. Spontaneous low-frequency bold signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 26, 15–29 (2005).
Fox, M.D. et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 102, 9673–9678 (2005).
Whitfield-Gabrieli, S. & Ford, J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 8, 49–76 (2012).
Murphy, K., Birn, R., Handwerker, D., Jones, T.B. & Bandettini, P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905 (2009).
Chai, X.J., Castañón, A.N., Öngür, D. & Whitfield-Gabrieli, S. Anticorrelations in resting state networks without global signal regression. Neuroimage 59, 1420–1428 (2012).
He, B.J., Snyder, A.Z., Zempel, J.M., Smyth, M.D. & Raichle, M.E. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc. Natl. Acad. Sci. USA 105, 16039–16044 (2008).
Wang, L., Saalmann, Y.B., Pinsk, M.A., Arcaro, M.J. & Kastner, S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron 76, 1010–1020 (2012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Resting-state functional connectivity of lateral parietal cortex. The intrinsic functional connectivity networks of human parietal cortex are displayed for a 4-mm seed region that is gradually moved along the cortical surface. The functional connectivity networks are estimated on the surface using resting-state functional MRI data from 1000 young adults. The seed region begins in a region at or near the human homologue of LIP and gradually moves through distinct parietal regions including those primarily coupled to limbic regions. Note that multiple interdigitated networks converge on contiguous regions of parietal cortex. Some of these regions are embedded in sensory-motor circuits; others lack coupling to sensory or motor regions and are embedded in networks comprising what has come to be known as the 'default network'. Thus, human parietal cortex represents a nexus of multiple, interdigitated association pathways. (AVI 8770 kb)
Supplementary Video 2
Resting-state functional connectivity of lateral parietal cortex. The left and right panels each show a 4-mm seed region that is gradually moved along identical trajectories through lateral temporal and parietal cortex. Left panel functional connectivity patterns are computed from a dataset of passive rest collected in 16 subjects. The right panel consists of data collected in the same participants during performance of a semantic classification task. (AVI 14645 kb)
Rights and permissions
About this article
Cite this article
Buckner, R., Krienen, F. & Yeo, B. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16, 832–837 (2013). https://doi.org/10.1038/nn.3423
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3423
This article is cited by
-
Atypical local brain connectivity in pediatric autism spectrum disorder? A coordinate-based meta-analysis of regional homogeneity studies
European Archives of Psychiatry and Clinical Neuroscience (2024)
-
Correspondence of functional connectivity gradients across human isocortex, cerebellum, and hippocampus
Communications Biology (2023)
-
CCA identifies a neurophysiological marker of adaptation capacity that is reliably linked to internal locus of control of cognition in amnestic MCI
GeroScience (2023)
-
Exercise alters cortico-basal ganglia network metabolic connectivity: a mesoscopic level analysis informed by anatomic parcellation defined in the mouse brain connectome
Brain Structure and Function (2023)
-
Meta-analytic evidence that mindfulness training alters resting state default mode network connectivity
Scientific Reports (2022)