Abstract
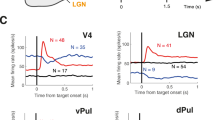
The primary visual cortex (V1) receives its driving input from the eyes via the lateral geniculate nucleus (LGN) of the thalamus. The lateral pulvinar nucleus of the thalamus also projects to V1, but this input is not well understood. We manipulated lateral pulvinar neural activity in prosimian primates and assessed the effect on supra-granular layers of V1 that project to higher visual cortex. Reversibly inactivating lateral pulvinar prevented supra-granular V1 neurons from responding to visual stimulation. Reversible, focal excitation of lateral pulvinar receptive fields increased the visual responses in coincident V1 receptive fields fourfold and shifted partially overlapping V1 receptive fields toward the center of excitation. V1 responses to regions surrounding the excited lateral pulvinar receptive fields were suppressed. LGN responses were unaffected by these lateral pulvinar manipulations. Excitation of lateral pulvinar after LGN lesion activated supra-granular layer V1 neurons. Thus, lateral pulvinar is able to powerfully control and gate information outflow from V1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kandel, E.R., Schwartz, J.H. & Jessell, T.M. Principles of Neural Science (New York, McGraw-Hill, 2000).
Purves, D., Augustine, G.J. & Fitzpatrick, D. Neurosciencez (Sinauer Associates, 2004).
Van Essen, D.C., Anderson, C.H. & Felleman, D.J. Information processing in the primate visual system: an integrated systems perspective. Science 255, 419–423 (1992).
Felleman, D.J. & Van Essen, D.C. Distributed hierarchical processing in primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991).
Kaas, J.H. & Lyon, D.C. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res. Rev. 55, 285–296 (2007).
Robinson, D.L. & Robinson, S.E. Pulvinar and visual salience. Trends Neurosci. 15, 127–132 (1992).
Casanova, C. The visual functions of the pulvinar. in The Visual Neurosciences (eds. Werner, J.S. & Chalupa, L.M.) 592–608 (Cambridge, Massachusetts, MIT Press, 2004).
Benevento, L.A. & Rezak, M. The cortical projections of the inferior pulvinar and adjacent lateral pulvinar in the rhesus monkey (Macaca mulatta): an autoradiographic study. Brain Res. 108, 1–24 (1976).
Rezak, M. & Benevento, L.A. A comparison of the projections of the dorsal lateral geniculate nucleus, the inferior pulvinar and adjacent lateral pulvinar to striate cortex (area 17) in the macaque monkey. Brain Res. 167, 19–40 (1979).
Ogren, M.P. & Hendrickson, A.E. The distribution of pulvinar terminals in visual areas 17 and 18 of the monkey. Brain Res. 137, 343–350 (1977).
Crick, F. & Koch, C. Constraints on cortical and thalamic projections: the no-strong-loops hypothesis. Nature 391, 245–250 (1998).
Ward, R., Danziger, S., Owen, V. & Rafal, R. Deficits in spatial coding and feature binding following damage to the human pulvinar. Nat. Neurosci. 5, 99–100 (2002).
Karnath, H.-O., Himmelbach, M. & Rorden, C. The subcortical anatomy of human spatial neglect: putamen, caudate nucleus, and pulvinar. Brain 125, 350–360 (2002).
Arend, I. et al. The role of the human pulvinar in visual attention and action: evidences from temporal order judgment, saccade decision and anti-saccade tasks. Prog. Brain Res. 171, 475–483 (2008).
Snow, J.C., Allen, H.A., Rafal, R.D. & Humphreys, G.W. Impaired attentional selection following lesions to human pulvinar: evidence for homology between human and monkey. Proc. Natl. Acad. Sci. USA 106, 4054–4059 (2009).
Chalupa, L.M. A review of cat and monkey studies implicating the pulvinar in visual function. Behav. Biol. 20, 149–167 (1977).
Ungerleider, L.G. & Christensen, C.A. Pulvinar lesions in monkeys produce abnormal scanning of a complex visual array. Neuropsychologia 17, 493–501 (1979).
Bender, D.B. & Butter, C.M. Comparison of the effects of superior colliculus and pulvinar lesions on visual search and tachistoscopic pattern discrimination in monkeys. Exp. Brain Res. 69, 140–154 (1987).
Rafal, R.D. & Posner, M.I. Deficits in human visual spatial attention following thalamic lesions. Proc. Natl. Acad. Sci. USA 84, 7349–7353 (1987).
Wilke, M., Turchi, J., Smith, K., Mishkin, M. & Leopold, D.A. Pulvinar inactivation disrupts selection of movement plans. J. Neurosci. 30, 8650–8659 (2010).
Fischer, J. & Whitney, D. Precise discrimination of object position in the human pulvinar. Hum. Brain Mapp. 30, 101–111 (2009).
Smith, A.T., Cotton, P.L., Bruno, A. & Moutsiana, C. Dissociating vision and visual attention in the human pulvinar. J. Neurophysiol. 101, 917–925 (2009).
Desimone, R., Wessinger, M., Thomas, L. & Schneider, W. Attentional control of visual perception: cortical and subcortical mechanisms. Cold Spring Harb. Symp. Quant. Biol. 55, 963–971 (1990).
Petersen, S.E., Robinson, D.L. & Morris, J.D. Contributions of the pulvinar to visual spatial attention. Neuropsychologia 25, 97–105 (1987).
Bender, D.B. & Youakim, M. The effect of attentive fixation in macaque thalamus and cortex. J. Neurophysiol. 85, 219–234 (2001).
Kastner, S. et al. Functional imaging of the human lateral geniculate nucleus and pulvinar. J. Neurophysiol. 91, 438–448 (2004).
Wilke, M., Mueller, K.M. & Leopold, D.A. Neural activity in the visual thalamus reflects perceptual suppression. Proc. Natl. Acad. Sci. USA 106, 9465–9470 (2009).
Berman, R.A. & Wurtz, R.H. Signals conveyed in the pulvinar pathway from superior colliculus to cortical area MT. J. Neurosci. 31, 373–384 (2011).
Ivanov, I. et al. Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am. J. Psychiatry 167, 397–408 (2010).
Mize, R.R. & White, D.A. [3H]Muscimol labels neurons in both the superficial and deep layers of cat superior colliculus. Neurosci. Lett. 104, 31–37 (1989).
Martin, J.H. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci. Lett. 127, 160–164 (1991).
Hikosaka, O. & Wurtz, R.H. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J. Neurophysiol. 53, 292–308 (1985).
Reid, R.C. & Alonso, J.M. Specificity of monosynaptic connections from thalamus to visual cortex. Nature 378, 281–284 (1995).
Molotchnikoff, S. & Shumikhina, S. The lateral posterior-pulvinar complex modulation of stimulus-dependent oscillations in the cat visual cortex. Vision Res. 36, 2037–2046 (1996).
Logothetis, N.K. et al. The effects of electrical microstimulation on cortical signal propagation. Nat. Neurosci. 13, 1283–1291 (2010).
Sherman, S.M. & Guillery, R.W. Distinct functions for direct and transthalamic corticocortical connections. J. Neurophysiol. 106, 1068–1077 (2011).
De Pasquale, R. & Sherman, S.M. Synaptic properties of corticocortical connections between the primary and secondary visual cortical areas in the mouse. J. Neurosci. 31, 16494–16506 (2011).
McCormick, D.A., Shu, Y.S. & Hasenstaub, A. Balanced recurrent excitation and inhibition in local cortical networks. in Excitatory-Inhibitory Balance: Synapses, Circuits, Systems (ed. Hensch, T.) 113–124 (Kluver Academic Press, New York, 2003).
Ferster, D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J. Neurosci. 6, 1284–1301 (1986).
Softky, W.R. & Koch, C. The highly irregular firing of cortical cells is inconsistent with temporal integration of random EPSPs. J. Neurosci. 13, 334–350 (1993).
Borg-Graham, L.J., Monier, C. & Fregnac, Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature 393, 369–373 (1998).
Somers, D.C., Nelson, S.B. & Sur, M. An emergent model of orientation selectivity in cat visual cortical simple cells. J. Neurosci. 15, 5448–5465 (1995).
Mariño, J. et al. Invariant computations in local cortical networks with balanced excitation and inhibition. Nat. Neurosci. 8, 194–201 (2005).
Callaway, E.M. Local circuits in primary visual cortex of the macaque monkey. Annu. Rev. Neurosci. 21, 47–74 (1998).
Rockland, K.S. Convergence and branching patterns of round, type 2 corticopulvinar axons. J. Comp. Neurol. 390, 515–536 (1998).
Desimone, R. & Duncan, J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18, 193–222 (1995).
Van Essen, D.C. Cortico-cortical and thalamo-cortical information flow in the primate visual system. in Cortical Function: A View from the Thalamus (eds. Casagrande, V.A., Guillery, R. & Sherman, M.) 173–185 (Elsevier, 2005).
Olshausen, B.A., Anderson, C.H. & Van Essen, D.C. A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. J. Neurosci. 13, 4700–4719 (1993).
Ramachandran, V.S. & Gregory, R. Perceptual filling in of artificially induced scotomas in human vision. Nature 350, 699–702 (1991).
Bishop, P.O., Henry, G.H. & Smith, C.J. Binocular interaction fields of single units in the cat striate cortex. J. Physiol. 216, 39–68 (1971).
Karnovsky, M.J. & Roots, L.A. 'direct coloring' thiocholine method for cholinesterases. J. Histochem. Cytochem. 12, 219–221 (1964).
Acknowledgements
We thank J. Mavity-Hudson for histology, S. Walston, Y. Jiang, D. Yampolsky, J. Mavity-Hudson, J. Patel and D. Rinker for help with experiments, D. Yampolsky for technical and computational assistance, and M. Feurtado for veterinary assistance. We thank F. Ebner, J. Kaas, R. Khumbani, J. Schall, P. Wallisch and K.-W. Yao for their suggestions and comments on the work. This work was supported by US National Institutes of Health grants R01-EY01778, P30-EY008126, T32-EY07135 and P30-HD15052.
Author information
Authors and Affiliations
Contributions
G.P. and V.A.C. designed the experiments. G.P., R.M., K.L. and V.A.C. performed the experiments. G.P. analyzed the data and wrote the paper. G.P., R.M., K.L. and V.A.C. edited the manuscript. V.A.C. supervised the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 4368 kb)
Rights and permissions
About this article
Cite this article
Purushothaman, G., Marion, R., Li, K. et al. Gating and control of primary visual cortex by pulvinar. Nat Neurosci 15, 905–912 (2012). https://doi.org/10.1038/nn.3106
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3106
This article is cited by
-
Sound suppresses earliest visual cortical processing after sight recovery in congenitally blind humans
Communications Biology (2024)
-
Long-range and cross-frequency neural modulation of gamma flicker on vigilance decrement
Cognitive Neurodynamics (2023)
-
Layer-specific, retinotopically-diffuse modulation in human visual cortex in response to viewing emotionally expressive faces
Nature Communications (2022)
-
From representations in predictive processing to degrees of representational features
Minds and Machines (2022)
-
Volume reduction without neuronal loss in the primate pulvinar complex following striate cortex lesions
Brain Structure and Function (2021)