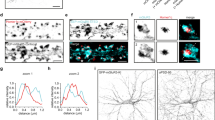
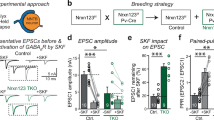
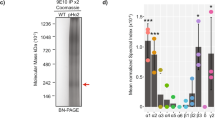
Abstract
The regulation of glycine receptor (GlyR) number at synapses is necessary for the efficacy of inhibition and the control of neuronal excitability in the spinal cord. GlyR accumulation at synapses depends on the scaffolding molecule gephyrin and is linked to GlyR synaptic dwell time. However, the mechanisms that tune GlyR synaptic exchanges in response to different neuronal environments are unknown. Integrins are cell adhesion molecules and signaling receptors. Using single quantum dot imaging and fluorescence recovery after photobleaching, we found in rats that β1 and β3 integrins adjust synaptic strength by regulating the synaptic dwell time of both GlyRs and gephyrin. β1 and β3 integrins crosstalked via calcium/calmodulin-dependent protein kinase II and adapted GlyR lateral diffusion and gephyrin-dependent trapping at synapses. This provides a mechanism for maintaining or adjusting the steady state of postsynaptic molecule exchanges and the level of glycinergic inhibition in response to neuron- and glia-derived signals or extracellular matrix remodeling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Triller, A. & Choquet, D. New concepts in synaptic biology derived from single-molecule imaging. Neuron 59, 359–374 (2008).
Calamai, M. et al. Gephyrin oligomerization controls GlyR mobility and synaptic clustering. J. Neurosci. 29, 7639–7648 (2009).
Gray, N.W., Weimer, R.M., Bureau, I. & Svoboda, K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 4, e370 (2006).
Specht, C.G. & Triller, A. The dynamics of synaptic scaffolds. Bioessays 30, 1062–1074 (2008).
Dahan, M. et al. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science 302, 442–445 (2003).
Hynes, R.O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Miranti, C.K. & Brugge, J.S. Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4, E83–E90 (2002).
Pinkstaff, J.K., Detterich, J., Lynch, G. & Gall, C. Integrin subunit gene expression is regionally differentiated in adult brain. J. Neurosci. 19, 1541–1556 (1999).
Chan, C.S., Weeber, E.J., Kurup, S., Sweatt, J.D. & Davis, R.L. Integrin requirement for hippocampal synaptic plasticity and spatial memory. J. Neurosci. 23, 7107–7116 (2003).
Chavis, P. & Westbrook, G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature 411, 317–321 (2001).
Cingolani, L.A. et al. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron 58, 749–762 (2008).
Kloss, C.U. et al. Integrin family of cell adhesion molecules in the injured brain: regulation and cellular localization in the normal and regenerating mouse facial motor nucleus. J. Comp. Neurol. 411, 162–178 (1999).
Staubli, U., Vanderklish, P. & Lynch, G. An inhibitor of integrin receptors blocks long-term potentiation. Behav. Neural Biol. 53, 1–5 (1990).
Chan, C.S. et al. Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity and working memory. J. Neurosci. 26, 223–232 (2006).
Huang, Z. et al. Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J. Neurosci. 26, 11208–11219 (2006).
Kramár, E.A., Lin, B., Rex, C.S., Gall, C.M. & Lynch, G. Integrin-driven actin polymerization consolidates long-term potentiation. Proc. Natl. Acad. Sci. USA 103, 5579–5584 (2006).
Betz, H. & Laube, B. Glycine receptors: recent insights into their structural organization and functional diversity. J. Neurochem. 97, 1600–1610 (2006).
Pfaff, M., McLane, M.A., Beviglia, L., Niewiarowski, S. & Timpl, R. Comparison of disintegrins with limited variation in the RGD loop in their binding to purified integrins alpha IIb beta 3, alpha V beta 3 and alpha 5 beta 1 and in cell adhesion inhibition. Cell Adhes. Commun. 2, 491–501 (1994).
Wang, X.B. et al. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc. Natl. Acad. Sci. USA 105, 19520–19525 (2008).
Charrier, C., Ehrensperger, M.V., Dahan, M., Levi, S. & Triller, A. Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. J. Neurosci. 26, 8502–8511 (2006).
Lévi, S. et al. Homeostatic regulation of synaptic GlyR numbers driven by lateral diffusion. Neuron 59, 261–273 (2008).
Hanus, C., Ehrensperger, M.V. & Triller, A. Activity-dependent movements of postsynaptic scaffolds at inhibitory synapses. J. Neurosci. 26, 4586–4595 (2006).
DeFreitas, M.F. et al. Identification of integrin alpha 3 beta 1 as a neuronal thrombospondin receptor mediating neurite outgrowth. Neuron 15, 333–343 (1995).
Möller, J.C. et al. Regulation of thrombospondin in the regenerating mouse facial motor nucleus. Glia 17, 121–132 (1996).
Lin, T.N. et al. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke 34, 177–186 (2003).
Schachtrup, C. et al. Fibrinogen inhibits neurite outgrowth via beta 3 integrin-mediated phosphorylation of the EGF receptor. Proc. Natl. Acad. Sci. USA 104, 11814–11819 (2007).
Hanus, C., Vannier, C. & Triller, A. Intracellular association of glycine receptor with gephyrin increases its plasma membrane accumulation rate. J. Neurosci. 24, 1119–1128 (2004).
Ehrensperger, M.V., Hanus, C., Vannier, C., Triller, A. & Dahan, M. Multiple association states between glycine receptors and gephyrin identified by SPT analysis. Biophys. J. 92, 3706–3718 (2007).
Bedet, C. et al. Regulation of gephyrin assembly and glycine receptor synaptic stability. J. Biol. Chem. 281, 30046–30056 (2006).
Zita, M.M. et al. Post-phosphorylation prolyl isomerisation of gephyrin represents a mechanism to modulate glycine receptors function. EMBO J. 26, 1761–1771 (2007).
Wu, X. et al. Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands. J. Cell Biol. 143, 241–252 (1998).
Blystone, S.D., Slater, S.E., Williams, M.P., Crow, M.T. & Brown, E.J. A molecular mechanism of integrin crosstalk: alphavbeta3 suppression of calcium/calmodulin-dependent protein kinase II regulates alpha5beta1 function. J. Cell Biol. 145, 889–897 (1999).
Steiner, P. et al. Destabilization of the postsynaptic density by PSD-95 serine 73 phosphorylation inhibits spine growth and synaptic plasticity. Neuron 60, 788–802 (2008).
Hayashi, M.K. et al. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 137, 159–171 (2009).
Trinidad, J.C., Specht, C.G., Thalhammer, A., Schoepfer, R. & Burlingame, A.L. Comprehensive identification of phosphorylation sites in postsynaptic density preparations. Mol. Cell. Proteomics 5, 914–922 (2006).
Tweedie-Cullen, R.Y., Reck, J.M. & Mansuy, I.M. Comprehensive mapping of post-translational modifications on synaptic, nuclear and histone proteins in the adult mouse brain. J. Proteome Res. 8, 4966–4982 (2009).
Saiyed, T. et al. Molecular basis of gephyrin clustering at inhibitory synapses: role of G- and E-domain interactions. J. Biol. Chem. 282, 5625–5632 (2007).
Liauw, J. et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J. Cereb. Blood Flow Metab. 28, 1722–1732 (2008).
Woolf, C.J. & Salter, M.W. Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000).
Harvey, R.J. et al. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 304, 884–887 (2004).
Grillner, S. The motor infrastructure: from ion channels to neuronal networks. Nat. Rev. Neurosci. 4, 573–586 (2003).
Kaneko, M., Stellwagen, D., Malenka, R.C. & Stryker, M.P. Tumor necrosis factor–alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron 58, 673–680 (2008).
Stellwagen, D. & Malenka, R.C. Synaptic scaling mediated by glial TNF-alpha. Nature 440, 1054–1059 (2006).
Lévi, S., Vannier, C. & Triller, A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters. J. Cell Sci. 111, 335–345 (1998).
Meier, J., Meunier-Durmort, C., Forest, C., Triller, A. & Vannier, C. Formation of glycine receptor clusters and their accumulation at synapses. J. Cell Sci. 113, 2783–2795 (2000).
Dumoulin, A., Levi, S., Riveau, B., Gasnier, B. & Triller, A. Formation of mixed glycine and GABAergic synapses in cultured spinal cord neurons. Eur. J. Neurosci. 12, 3883–3892 (2000).
Saxton, M.J. Single-particle tracking: the distribution of diffusion coefficients. Biophys. J. 72, 1744–1753 (1997).
Kusumi, A., Sako, Y. & Yamamoto, M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys. J. 65, 2021–2040 (1993).
Meier, J., De Chaldee, M., Triller, A. & Vannier, C. Functional heterogeneity of gephyrins. Mol. Cell. Neurosci. 16, 566–577 (2000).
Paarmann, I., Schmitt, B., Meyer, B., Karas, M. & Betz, H. Mass spectrometric analysis of glycine receptor-associated gephyrin splice variants. J. Biol. Chem. 281, 34918–34925 (2006).
Acknowledgements
We thank members of the Triller laboratory, S. Supplisson, K. Aubrey, L. Wang and O. Pascual for kindly providing access to their patch-clamp rig and for helpful discussions, Y. Goda and L. Cingolani for integrin constructs and C. Specht, B. Barbour and R. Miles for critical reading of the manuscript. This work was supported by INSERM (A.T.), Agence Nationale de la Recherche (ANR08BLAN0282; A.T.), Fondation Pierre-Gilles de Gennes (A.T.), Institut pour la Recherche sur la Moelle épinière et l'Encéphale (A.T.), Ministère de la Recherche et de la Technologie (C.C. and P.M.) and Association Française contre les Myopathies (C.C.).
Author information
Authors and Affiliations
Contributions
C.C. designed, performed and analyzed the experiments except for the in vitro phosphorylation assays and mass spectrometry and wrote the manuscript with help from the other authors. P.M. performed the in vitro phosphorylation assays. R.Y.T.-C. and D.R. performed mass spectrometry and analyzed data. I.M.M. supervised mass spectrometry and phosphorylation analyses. A.T. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 3339 kb)
Rights and permissions
About this article
Cite this article
Charrier, C., Machado, P., Tweedie-Cullen, R. et al. A crosstalk between β1 and β3 integrins controls glycine receptor and gephyrin trafficking at synapses. Nat Neurosci 13, 1388–1395 (2010). https://doi.org/10.1038/nn.2645
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2645
This article is cited by
-
GABAergic synapses onto SST and PV interneurons in the CA1 hippocampal region show cell-specific and integrin-dependent plasticity
Scientific Reports (2023)
-
Integrin β3 in forebrain Emx1-expressing cells regulates repetitive self-grooming and sociability in mice
BMC Neuroscience (2022)
-
Cadherin-13 is a critical regulator of GABAergic modulation in human stem-cell-derived neuronal networks
Molecular Psychiatry (2022)
-
How the epigenome integrates information and reshapes the synapse
Nature Reviews Neuroscience (2019)
-
Osteopontin counters human immunodeficiency virus type 1–induced impairment of neurite growth through mammalian target of rapamycin and beta-integrin signaling pathways
Journal of NeuroVirology (2019)