Abstract
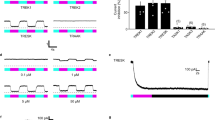
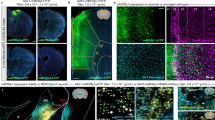
Primary nociceptors are the first neurons involved in the complex processing system that regulates normal and pathological pain1. Because of constraints on pharmacological and electrical stimulation, noninvasive excitation and inhibition of these neurons in freely moving nontransgenic animals has not been possible. Here we use an optogenetic2 strategy to bidirectionally control nociceptors of nontransgenic mice. Intrasciatic nerve injection of adeno-associated viruses encoding an excitatory opsin enabled light-inducible stimulation of acute pain, place aversion and optogenetically mediated reductions in withdrawal thresholds to mechanical and thermal stimuli. In contrast, viral delivery of an inhibitory opsin enabled light-inducible inhibition of acute pain perception, and reversed mechanical allodynia and thermal hyperalgesia in a model of neuropathic pain. Light was delivered transdermally, allowing these behaviors to be induced in freely moving animals. This approach may have utility in basic and translational pain research, and enable rapid drug screening and testing of newly engineered opsins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dubin, A.E. & Patapoutian, A. Review series: nociceptors: the sensors of the pain pathway. J. Clin. Invest. 120, 3760–3772 (2010).
Fenno, L., Yizhar, O. & Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412 (2011).
Liske, H. et al. Optical inhibition of motor nerve and muscle activity in vivo. Muscle Nerve 47, 916–921 (2013).
Llewellyn, M.E., Thompson, K.R., Deisseroth, K. & Delp, S.L. Orderly recruitment of motor units under optical control in vivo. Nat. Med. 16, 1161–1165 (2010).
Ji, Z.-G. et al. Light-evoked somatosensory perception of transgenic rats that express channelrhodopsin-2 in dorsal root ganglion cells. PLoS ONE 7, e32699 (2012).
Wang, H. & Zylka, M.J. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J. Neurosci. 29, 13202–13209 (2009).
Mourot, A. et al. Rapid optical control of nociception with an ion-channel photoswitch. Nat. Methods 9, 396–402 (2012).
Kokel, D. et al. Photochemical activation of TRPA1 channels in neurons and animals. Nat. Chem. Biol. 9, 257–263 (2013).
Anonymous. Enlightened engineering. Nat. Biotechnol. 29, 849 (2011).
Towne, C., Montgomery, K.L., Iyer, S.M., Deisseroth, K. & Delp, S.L. Optogenetic control of targeted peripheral axons in freely moving animals. PLoS ONE 8, e72691 (2013).
Daou, I. et al. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J. Neurosci. 33, 18631–18640 (2013).
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 (2012).
Williams, J.C. & Denison, T. From optogenetic technologies to neuromodulation therapies. Sci. Transl. Med. 5, 177ps6 (2013).
Chow, B.Y. & Boyden, E.S. Optogenetics and translational medicine. Sci. Transl. Med. 5, 177ps5 (2013).
Towne, C., Schneider, B.L., Kieran, D., Redmond, D.E. & Aebischer, P. Efficient transduction of non-human primate motor neurons after intramuscular delivery of recombinant AAV serotype 6. Gene Ther. 17, 141–146 (2010).
San Sebastian, W. et al. Adeno-associated virus type 6 is retrogradely transported in the non-human primate brain. Gene Ther. 20, 1178–1183 (2013).
Asokan, A., Schaffer, D.V. & Samulski, R.J. The AAV vector toolkit: poised at the clinical crossroads. Mol. Ther. 20, 699–708 (2012).
Towne, C., Pertin, M., Beggah, A.T., Aebischer, P. & Decosterd, I. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol. Pain 5, 52 (2009).
Mogil, J.S. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 10, 283–294 (2009).
Mason, M.R.J. et al. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol. Ther. 18, 715–724 (2010).
Bennett, G.J. & Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33, 87–107 (1988).
Lin, J.Y., Knutsen, P.M., Muller, A., Kleinfeld, D. & Tsien, R.Y. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat. Neurosci. 16, 1499–1508 (2013).
Bashkatov, A.N., Genina, E.A., Kochubey, V.I. & Tuchin, V.V. Optical properties of human skin, subcutaneous and mucous tissues in the wavelength range from 400 to 2000 nm. J. Phys. D Appl. Phys. 38, 2543–2555 (2005).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Konermann, S. et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature 500, 472–476 (2013).
Sohal, V.S., Zhang, F., Yizhar, O. & Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 698–702 (2009).
Tillman, D.B., Treede, R.D., Meyer, R.A. & Campbell, J.N. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: estimates of receptor depth and threshold. J. Physiol. (Lond.) 485, 753–765 (1995).
Gao, G.-P. et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 99, 11854–11859 (2002).
Lee, J.H. et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 (2010).
Kügler, S., Kilic, E. & Bähr, M. Human synapsin 1 gene promoter confers highly neuron-specific long-term transgene expression from an adenoviral vector in the adult rat brain depending on the transduced area. Gene Ther. 10, 337–347 (2003).
Gold, M.S. Whole-cell recording in isolated primary sensory neurons. Methods Mol. Biol. 851, 73–97 (2012).
Hentschke, H. & Stüttgen, M.C. Computation of measures of effect size for neuroscience data sets. Eur. J. Neurosci. 34, 1887–1894 (2011).
Chaplan, S.R., Bach, F.W., Pogrel, J.W., Chung, J.M. & Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 20, 441–462 (1980).
Sommer, C. & Schäfers, M. Painful mononeuropathy in C57BL/Wld mice with delayed Wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 784, 154–162 (1998).
Acknowledgements
We thank H. Liske, X. Qian, S. Mackey, A. Weitz, D.J. Clark, D.C. Yeomans, P. Sabhaie, C. Gorini, S. Young and H. Scutt for useful discussions and assistance with experiments. This study was supported by the US National Institutes of Health (National Institute of Neurological Disorders and Stroke grant R01-NS080954), the Stanford Bio-X NeuroVentures program and the Stanford Bio-X Interdisciplinary Initiatives program. S.M.I. was supported by an Office of Technology Licensing Stanford Graduate Fellowship and by a Howard Hughes Medical Institute International Student Research Fellowship. K.L.M. was supported by a Bio-X Bioengineering Graduate Fellowship and by a Stanford Interdisciplinary Graduate Fellowship. C.T. was supported by a Swiss National Science Foundation Fellowship.
Author information
Authors and Affiliations
Contributions
S.M.I., K.L.M., C.T. and S.L.D. designed the experiments. S.M.I. and K.L.M. performed the experiments. C.R. performed cell culture and created vectors. S.Y.L. performed electrophysiology. K.D. contributed reagents and tools. S.M.I., K.L.M. and S.L.D. wrote and edited the paper, with comments from all other authors.
Corresponding author
Ethics declarations
Competing interests
C.T., K.D., and S.L.D. have a financial interest in Circuit Therapeutics, Inc., which, however, did not support this work. S.M.I., K.L.M., C.T., K.D. and S.L.D. have disclosed these findings to the Stanford Office of Technology Licensing for potential use in the identification of new treatments for pain.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 4611 kb)
Supplementary Video 1
Video demonstration of transdermal optogenetic activation of nociceptors. A mouse with bilateral ChR2 expression is placed in a clear cylinder on a transparent glass plate, and allowed to habituate to its environment. When blue light (473 nm, 1 mW/mm2) is shone on the plantar surface of the mouse's skin it immediately withdraws its paw, engages in prolonged licking behavior, and shakes its paw. However, when yellow light (593 nm, 1 mW/mm2) is shone on the skin, there is no observable change in behavior, and the mouse moves around normally. Also, when blue light (473 nm, 1 mW/mm2) is shone on the paw of a bilaterally injected YFP+ mouse, no change in behavior is seen, and the mouse moves around normally. (MOV 8136 kb)
Rights and permissions
About this article
Cite this article
Iyer, S., Montgomery, K., Towne, C. et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol 32, 274–278 (2014). https://doi.org/10.1038/nbt.2834
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.2834
This article is cited by
-
A photoswitchable inhibitor of TREK channels controls pain in wild-type intact freely moving animals
Nature Communications (2023)
-
Fatigue-resistant hydrogel optical fibers enable peripheral nerve optogenetics during locomotion
Nature Methods (2023)
-
Optogenetic stimulation of vagal nerves for enhanced glucose-stimulated insulin secretion and β cell proliferation
Nature Biomedical Engineering (2023)
-
Towards translational optogenetics
Nature Biomedical Engineering (2022)
-
Optogenetic Stimulation of the Anterior Cingulate Cortex Modulates the Pain Processing in Neuropathic Pain: A Review
Journal of Molecular Neuroscience (2022)