Abstract
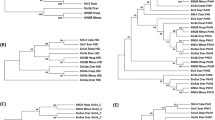
Caenorhabditis elegans is the first animal whose genomic sequence has been determined1. One of the new possibilities in post-sequence genetics is the analysis of complete gene families at once. We studied the family of heterotrimeric G proteins2. C. elegans has 20 Gα, 2 Gβ and 2 Gγ genes. There is 1 homologue of each of the 4 mammalian classes of Gα genes, Gi/Goα, Gsα, Gqα and G12α, and there are 16 new α genes. Although the conserved Gα subunits are expressed in many neurons and muscle cells3,4,5,6,7, GFP fusions indicate that 14 new Gα genes are expressed almost exclusively in a small subset of the chemosensory neurons of C. elegans8,9. We generated loss-of-function alleles using target-selected gene inactivation10,11. None of the amphid-expressed genes are essential for viability, and only four show any detectable phenotype (chemotaxis defects), suggesting extensive functional redundancy. On the basis of functional analysis, the 20 genes encoding Gα proteins can be divided into two groups: those that encode subunits affecting muscle activity (homologues of Gi/Goα, Gsα and Gq; refs 3,4,5,6), and those (14 new genes) that encode proteins most likely involved in perception.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282, 2012–2018 (1998).
Neer, E.J. Heterotrimeric G proteins: organizers of transmembrane signals. Cell 80, 249–257 ( 1995).
Mendel, J.E. et al. Participation of the Go protein in multiple aspects of behavior in C. elegans. Science 267, 1652–1655 (1995).
Ségalat, L., Elkes, D.A. & Kaplan, J.M. Modulation of serotonin controlled behaviors by G o in Caenorhabditis elegans. Science 267 , 1648–1651 (1995).
Brundage, L. et al. Mutations in a C. elegans Gqα gene disrupt movement, egg-laying and viability. Neuron 16, 999–1009 (1996).
Korswagen, H.C., Park, J-H., Ohshima, Y. & Plasterk, R.H.A. An activating mutation in a Caenorhabditis elegans Gs protein induces neuronal degeneration. Genes Dev. 11, 1493 –1503 (1997).
Park, J-H., Ohshima, S., Tani, T. & Ohshima, Y. Structure and expression of the gsa-1 gene encoding a G protein α (s) subunit in C. elegans. Gene 194, 183– 190 (1997).
Zwaal, R.R., Mendel, J.E., Sternberg, P.W. & Plasterk, R.H.A. Two neuronal G proteins are involved in the chemosensation of dauer inducing pheromone by C. elegans. Genetics 145, 715–727 (1997).
Roayaie, K., Gage Crump, J., Sagasti, A. & Bargmann, C.I. The Gα protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron 20, 55–67 ( 1998).
Zwaal, R.R., Broeks, A., van Meurs, J., Groenen, J.T.M. & Plasterk, R.H.A. Target selected gene inactivation by using a frozen transposon insertion mutant bank. Proc. Natl Acad. Sci. USA 90, 7431–7435 ( 1993).
Jansen, G., Hazendonk, E., Thijssen, K.L. & Plasterk, R.H.A. Reverse genetics by chemical mutagenesis in Caenorhabditis elegans. Nature Genet. 17, 119– 121 (1997).
Lochrie, M.A., Mendel, J.E., Sternberg, P.W. & Simon, M.I. Homologous and unique G protein α subunits in the nematode Caenorhabditis elegans. Cell Regul. 2, 135– 154 (1991).
Zwaal, R.R. et al. G proteins are required for spatial orientation of early cell cleavage in C. elegans embryos. Cell 86 , 619–629 (1996).
Bargmann, C.I. & Mori, I. Chemotaxis and thermotaxis. in C. elegans II (eds Riddle, D.L., Blumenthal, T., Meyer, B.J. & Priess, J.R.) 717–737 (Cold Spring Harbor Press, New York, 1997).
White, J.G., Southgate, E., Thomson, J.N. & Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans . Philos. Trans. R. Soc. Lond. 314, 1–340 (1986).
Perkins, L.A., Hedgecock, E.M., Thomson, J.N. & Culotti, J.G. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117, 456–487 (1986).
Ward, S. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc. Natl Acad. Sci. USA 70, 817–821 (1973).
Culotti, J.G. & Russell, R.L. Osmotic avoidance defective mutants of the nematode Caenorhabditis elegans. Genetics 90, 243–256 (1978).
Bargmann, C.I., Hartwieg, E. & Horvitz, H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74, 515– 527 (1993).
Sengupta, P., Chou, J.H. & Bargmann, C.I. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell 84, 899–909 ( 1994).
Troemel, E.R., Kimmel, B.E. & Bargmann, C.I. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91, 161–169 (1997).
Buck, L.B. Information coding in the vertebrate olfactory system. Annu. Rev. Neurosci. 19, 517–544 ( 1996).
Kaplan, J.M. & Horvitz, H.R. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 90, 2227–2231 ( 1993).
Colbert, H.A. & Bargmann, C.I. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14, 803–812 (1995).
Troemel, E.R., Chou, J.H., Dwyer, N.D., Colbert, H.A. & Bargmann, C.I. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83 , 207–218 (1995).
Yu, S., Avery, L., Baude, E. & Garbers, D.L. Gyanylyl cyclase expression in specific sensory neurons: a new family of chemosensory receptors. Proc. Natl Acad. Sci. USA 94, 3384– 3387 (1997).
Altschul, S.F., Gish, W., Miller, W., Myers, E.W. & Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 ( 1990).
Eeckman, F.H. & Durbin, R. ACeDB and Macace. Methods Cell Biol. 48, 583–605 ( 1995).
Han, M. & Sternberg, P.W. Analysis of dominant negative mutations of the Caenorhabditis elegans let-60 ras gene. Genes Dev. 5, 2188–2198 (1991).
Bargmann, C.I. & Horvitz, H.R. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7, 729– 742 (1991).
Acknowledgements
We thank C. Bargmann for strains and help in identification of amphid neurons; L. Brundage and M. Simon for the communication of unpublished results; A. Coulson for cosmids; the Fire lab for GFP-expression vectors; R. Zwaal, J. Neels, R. Korswagen and Y. Kato for the isolation of mutant strains; C. de Vries for help with behavioural assays; S. Wicks for help with statistical analyses and behavioural assays; and C. van den Berg, P. Borst, R. Korswagen and S. Wicks for comments on the manuscript. This work was supported by grant NKI 94-809 from the Netherlands Cancer Foundation, by grant NWO-GMW 90104094 from the Netherlands Organization for Scientific Research, by grant 940-70-008 from the New Drugs Research Foundation to R.H.A.P., and by a Biotechnology Research Training Grant from the European Commission (BIO4CT965072) to P.W.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jansen, G., Thijssen, K., Werner, P. et al. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat Genet 21, 414–419 (1999). https://doi.org/10.1038/7753
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/7753
This article is cited by
-
A local interplay between diffusion and intraflagellar transport distributes TRPV-channel OCR-2 along C. elegans chemosensory cilia
Communications Biology (2022)
-
A novel functional cross-interaction between opioid and pheromone signaling may be involved in stress avoidance in Caenorhabditis elegans
Scientific Reports (2020)
-
Bright split red fluorescent proteins for the visualization of endogenous proteins and synapses
Communications Biology (2019)
-
Deciphering and modulating G protein signalling in C. elegans using the DREADD technology
Scientific Reports (2016)
-
CRF-like receptor SEB-3 in sex-common interneurons potentiates stress handling and reproductive drive in C. elegans
Nature Communications (2016)