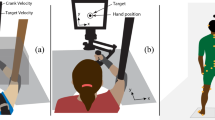
Abstract
The otoliths are stimulated in the same fashion by gravitational and inertial forces, so otolith signals are ambiguous indicators of self-orientation. The ambiguity can be resolved with added visual information indicating orientation and acceleration with respect to the earth. Here we present a Bayesian model of the statistically optimal combination of noisy vestibular and visual signals. Likelihoods associated with sensory measurements are represented in an orientation/acceleration space. The likelihood function associated with the otolith signal illustrates the ambiguity; there is no unique solution for self-orientation or acceleration. Likelihood functions associated with other sensory signals can resolve this ambiguity. In addition, we propose two priors, each acting on a dimension in the orientation/acceleration space: the idiotropic prior and the no-acceleration prior. We conducted experiments using a motion platform and attached visual display to examine the influence of visual signals on the interpretation of the otolith signal. Subjects made pitch and acceleration judgments as the vestibular and visual signals were manipulated independently. Predictions of the model were confirmed: (1) visual signals affected the interpretation of the otolith signal, (2) less variable signals had more influence on perceived orientation and acceleration than more variable ones, and (3) combined estimates were more precise than single-cue estimates. We also show that the model can explain some well-known phenomena including the perception of upright in zero gravity, the Aubert effect, and the somatogravic illusion.

















Similar content being viewed by others
Notes
In the vestibular literature, it is conventional for the X, Y, and Z axes to correspond to forward, upward, and lateral, respectively. We have chosen to use axes that are conventional in the vision literature.
The noise would actually be 3d in X–Y–Z space, but here we consider it collapsed onto the Z–Y plane. Note that Eq. 3 is valid for any head-centric plane, but for simplicity we discuss it in terms of the Z–Y plane only.
This conversion requires the assumption that ||G|| = 9.81 m/s2. This can be thought of as a prior on ||G||. This prior also has some variability associated with it, and that will generate additional variability in the otolith likelihood.
Pitch is a circular variable and acceleration is not. So plots of the likelihood functions in pitch-acceleration space should be cylindrical plots. Each point on the cylinder has a position along the circumference that represents pitch and a position perpendicular to the circumference that represents acceleration. For simplicity, we show the unwrapped cylinders in our figures.
For simplicity, we do not consider separately kinesthetic and somatosensory signals because the forces that affect them are the same as the forces that drive the otoliths.
Note that any error in scaling the scene will lead to an error in the velocity and acceleration estimates.
The maximum angular acceleration, which occurred very briefly at the beginning of platform motion, was ∼12.5°/s2. We had to place the rotation axis in the floor of the platform to maximize the motion range. The axis was ∼100 cm beneath and ∼26 cm in front of the center of the subject’s head. Thus, the angular acceleration generated small tangential and centripetal acceleration at the head. These accelerations were quite small and had virtually no influence on the total gravitoinertial force profiles.
In pilot testing, we found that people experienced both forward translation and pitch when presented the non-visual stimuli. Thus, it was reasonable to ask subjects which of two intervals yielded greater perceived acceleration and which of two intervals yields greater perceived pitch. Nonetheless, the mere instruction to interpret the otolith signal one way (e.g., as pitch, not acceleration) does not mean subjects are capable of completely succeeding, which can lead both to bias (when they attribute some of the signal to the acceleration instead) and increased variability (when the amount they attribute to acceleration varies from trial to trial).
Note that the posterior is centered on non-zero inertial acceleration, which means that the model predicts a perceived inertial acceleration that is slightly greater than zero. To our knowledge, no one has examined whether the conditions that produce the Aubert effect also cause a perceived acceleration.
References
Adelson EH, Movshon JA (1982) Phenomenal coherence of moving visual patterns. Nature 300:523–525
Alais D, Burr D (2004) The ventriloquist effect results from near-optimal bimodal integration. Curr Biol 14:257–262
Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJ (1999) Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci 19:316–327
Angelaki DE, Shiakh AG, Green AM, Dickman JD (2004) Neurons compute internal models of the physical laws of motion. Nature 430:560–564
Berger DR (2003) Spectral texturing for real-time applications. In: Siggraph 2003 sketches and applications. ACM Press, New York. DOI 10.1145/095400.965509
Benson AJ, Hutt ECB, Brown SF (1989) Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med 60:205–213
Cheung B, Money K, Wright H, Bateman W (1995) Spatial disorientation - implicated accidents in Canadian forces, 1982–1992. Aviat Space Environ Med 66(6):579–585
Dichgans J, Held R, Young LR, Brandt T (1972) Moving visual scenes influence the apparent direction of gravity. Science 178:1217–1219
Einstein A (1907) Über das Relativitätsprinzip und die aus demselben gezogenen Folgerungen. Jahrbuch der Radioaktivität und Elektronik 4:411–462
Ercoline WR (1997) Classification of the USAF SD mishaps. In: Braithwaite MG, DeRoche SL, Alvarez EA, Reese MA (eds) Proceedings of the first tri-service conference on rotary-wing spatial disorientation: spatial disorientation in the operational rotary-wing environment. USAARL report no. 97-15
Ernst MO, Banks MS (2002) Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415:429–433
Gepshtein S, Banks MS (2003) Viewing geometry determines how vision and haptics combine in size perception. Curr Biol 13:483–488
Ghahramani Z, Wolpert DM, Jordan MI (1997) Computational models of sensorimotor integration. In: Morasso PG, Sanguineti V (eds) Self-organization, computational maps, and motor control. Elsevier, Amsterdam, pp 117–147
Gibson JJ (1950) The perception of the visual world. Houghton-Miffin, Boston
Gibson JJ (1966) The senses considered as perceptual systems. Houghton-Mifflin, Boston
Gillingham KK, Previc FH (1993) Spatial orientation in flight. AL-TR-1993–0022. Brooks Air Force Base, Armstrong Laboratory, Texas
Glasauer S (1995) Linear acceleration perception: frequency dependence of the hilltop illusion. Acta Otolaryngol Suppl 520:37–40
Glasauer S, Mittelstaedt H (1998) Perception of spatial orientation in microgravity. Brain Res Brain Res Rev 28(1–2):185–193
Goldberg J, Fernandez C (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol 34:635–660
Groen EL, Valenti Clari MSV, Hosman RJAW (2001) Evaluation of perceived motion during a simulated takeoff run. J Aircraft 38(4):600–606
Harris LR, Jenkin M, Zikovitz DC (2000a) Vestibular capture of the perceived distance of passive linear self motion. Arch Ital Biol 138(1):63–7
Harris LR, Jenkin M, Zikovitz DC (2000b) Visual and non-visual cues in the perception of linear self motion. Exp Brain Res 135:12–21
Helmholtz H (1866) Treatise on physiological optics. Ed. trans. JPC Southall. Thoemmes Press, Bristol, 2000
Hillis JM, Ernst MO, Banks MS, Landy MS (2002) Combining sensory information: mandatory fusion within, but not between, senses. Science 298:1627–1630
Howard IP (1982) Human visual orientation. Wiley, New York
Howard IP, Childerson L (1994) The contribution of motion, the visual frame, and visual polarity to sensations of body tilt. Perception 23:753–762
Kersten D, Mamassian P, Yuille A (2004) Object perception as Bayesian inference. Ann Rev Psychol 55:271–304
Knill DC, Saunders JA (2003) Do humans optimally integrate stereo and texture information for judgments of surface slant? Vis Res 43:2539–2558
Körding KP, Wolpert DM (2004) Bayesian integration in sensorimotor learning. Nature 427(6971):244–247
Landy MS, Kojima H (2001) Ideal cue combination for localizing texture-defined edges. J Opt Soc Am A Opt Image Sci Vis 18:2307–2320
Landy MS, Maloney LT, Johnston EB, Young M (1995) Measurement and modeling of depth cue combination: in defense of weak fusion. Vis Res 35:389–412
Lessard CS, Matthews R, Yauch D (2000) Effects of rotation on somatogravic illusions. IEEE Eng Med Biol Mag 19:59–65
Longuet-Higgins HC, Prazdny K (1980) The interpretation of a moving retinal image. Proc R Soc Lond B 208:385–397
Malcolm R, Melvill-Jones G (1970) A quantitative study of vestibular adaptation in humans. Acta Otolaryngol 70:126–135
Maybeck PS (1979) Stochastic models, estimation and control. Academic, NewYork
Merfeld DM, Zupan L, Peterka RJ (1999) Humans use internal models to estimate gravity and linear acceleration. Nature 398:615–618
Merfeld DM, Zupan LH, Gifford CA (2001) Neural processing of gravito-inertial cues in humans. II. Influence of the semicircular canals during eccentric rotation. J Neurophysiol 85(4):1648–1660
Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S (2005) Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual response during translation and tilt. J Neurophysiol 94:186–198
Mittelstaedt H (1983) A new solution to the problem of the subjective vertical. Naturwissenschaften 70(6):272–281
Mittelstaedt ML, Mittelstaedt H (2001) Idiothetic navigation in humans: estimation of path length. Exp Brain Res 139:318–332
Oruç I, Maloney LT, Landy MS (2003) Weighted linear cue combination with possibly correlated error. Vis Res 43:2451–2468
Otakeno S, Matthews RSJ, Folio L, Previc FH, Lessard CS (2002) The effects of visual scenes on roll and pitch thresholds in pilots versus nonpilots. Aviat Space Environ Med 73:98–101
Previc FH, Varner DC, Gillingham KK (1992) Visual scene effects on the somatogravic illusion. Aviat Space Environ Med 63:1060–1064
Reymond G, Kemeny A (2000) Motion cueing in the Renault driving simulator. Vehicle Syst Dynam 34(4):249–259
Reymond G, Droulez J, Kemeny A (2002) Visuovestibular perception of self-motion modeled as a dynamic optimization process. Biol Cybern 87(4):301–314
Roach NW, Heron J, McGraw PV (2006) Resolving multisensory conflict: a strategy for balancing the costs and benefits of audio-visual integration. Proc R Soc B Biol Sci 273(1598):2159–2168
Royden CS, Crowell JA, Banks MS (1994) Estimating heading during eye movements. Vis Res 34(23):3197–3214
Seidman SH, Telford L, Paige GD (1998) Tilt perception during dynamic linear acceleration. Exp Brain Res 119(3):307–314
Stocker AA, Simoncelli EP (2006) Noise characteristics and prior expectations in human visual speed perception. Nat Neurosci 9(4):578–585
Stone LS, Thompson P (1992) Human speed perception is contrast dependent. Vis Res 32(8):1535–1549
Tokumaru O, Kaida K, Ashida H, Mizumoto C, Tatsuno J (1998) Visual influence on magnitude of somatogravic illusion evoked on spatial disorientation demonstrator. Aviat Space Eviron Med 69:111–116
van Boxtel JJ, Wexler M, Droulez J (2003) Perception of plane orientation from self-generated and passively observed optic flow. J Vis 3(5):318–332
Weiss Y, Simoncelli EP, Adelson EH (2002) Motion illusions as optimal percepts. Nat Neurosci 5:598–604
Wexler M, Panerai F, Lamouret I, Droulez J (2001) Self-motion and the perception of stationary objects. Nature 409(6816):85–88
Wichmann FA, Hill NJ (2001) The psychometric function: I. Fitting, sampling, and goodness-of-fit. Perc Psychophys 63:1293–1313
Yuille AL, Bülthoff HH (1996) Bayesian decision theory and psychophysics. In: Richards W, Knill DC (eds) Perception as Bayesian inference. Cambridge University Press, Cambridge, pp 123–162
Zupan LH, Merfeld DM (2003) Neural processing of gravito-inertial cues in humans. IV. Influence of visual rotational cues during roll optokinetic stimuli. J Neurophysiol 89(1):390–400
Zupan LH, Merfeld DM (2005) Human ocular torsion and perceived roll responses to linear acceleration. J Vestib Res 15(4):173–183
Zupan LH, Merfeld DM, Darlot C (2002) Using sensory weighting to model the influence of canal, otolith and visual cues on spatial orientation and eye movements. Biol Cybern 86(3):209–230
Acknowledgments
This research was supported by NIH training grant (EY14194) to the Berkeley Vision Science program and AFOSR research grant (F49620) to Martin Banks. Thanks to MarcErnst for helpful discussion and the MPI workshop for techincal assistance. Special thanks to three reviewers who provided thoughtful and thorough comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MacNeilage, P.R., Banks, M.S., Berger, D.R. et al. A Bayesian model of the disambiguation of gravitoinertial force by visual cues. Exp Brain Res 179, 263–290 (2007). https://doi.org/10.1007/s00221-006-0792-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0792-0