Abstract
Rationale
Increases in cholinergic transmission are linked to depression in human subjects and animal models. We therefore examined the effect of decreasing nicotinic acetylcholine receptor (nAChR) activity in tests of antidepressant efficacy using C57BL/6J mice.
Objectives
We determined whether the noncompetitive nAChR antagonist mecamylamine had antidepressant-like effects in the forced swim test (FST) and tail suspension test (TST). These experiments were repeated in mice lacking either the β2- or α7-nAChR subunits to identify the nAChR subunits involved in mediating the antidepressant response to mecamylamine.
Materials and methods
Adult mice on the C57BL/6J background were acutely administered mecamylamine i.p. 30 min before testing in the FST or TST.
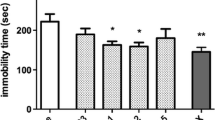
Results
A dose–response study showed that mecamylamine significantly decreased immobility time in the TST at the 1.0-mg/kg dose but did not alter baseline locomotor activity. The competitive nAChR antagonist dihydro-β-erythroidine, but not the blood–brain barrier impermeant antagonist hexamethonium, also decreased immobility in the TST. One milligram per kilogram of mecamylamine also significantly decreased time immobile in the FST whereas both β2- and α7-knockout mice were insensitive to the effects of mecamylamine in the FST.
Conclusions
Decreased activity of central nAChRs has antidepressant-like effects in both the TST and FST and these effects are dependent on both β2 and α7 subunits. Therefore, compounds that decrease nAChR activity may be attractive new candidates for development as antidepressants in humans.




Similar content being viewed by others
References
Breslau N (1995) Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet 25:95–101
Caldarone BJ, Karthigeyan K, Harrist A, Hunsberger JG, Wittmack E, King SL, Jatlow P, Picciotto MR (2003) Sex differences in response to oral amitriptyline in three animal models of depression in C57BL/6J mice. Psychopharmacology (Berl) 170:94–101
Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR (2004) High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry 56:657–664
Covey LS, Glassman AH, Stetner F (1997) Major depression following smoking cessation. Am J Psychiatry 154:263–265
Covey LS, Glassman AH, Stetner F (1998) Cigarette smoking and major depression. J Addict Dis 17:35–46
Crabbe JC, Wahlsten D, Dudek BC (1999) Genetics of mouse behavior: interactions with laboratory environment. Science 284:1670–1672
Crawley JN, Paylor R (1997) A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav 31:197–211
Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245
Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625
Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW (1998) Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr Res 33:113–118
Djuric VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M (1999) Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav 67:533–537
Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F (2000) Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacology (Berl) 152:295–303
Giniatullin R, Nistri A, Yakel JL (2005) Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci 28:371–378
Gosling JA, Lu TC (1969) Uptake and distribution of some quaternary ammonium compounds in the central nervous system of the rat. J Pharmacol Exp Ther 167:56–62
Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, Frederick S, Triffleman E (1998) Nortriptyline and cognitive–behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry 55:683–690
Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, Croghan IT, Glover ED, Sachs DP, Hurt RD (1999) Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br J Psychiatry 174:173–178
Janowsky DS, el-Yousef MK, Davis JM, Hubbard B, Sekerke HJ (1972a) Cholinergic reversal of manic symptoms. Lancet 1:1236–1237
Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ (1972b) A cholinergic–adrenergic hypothesis of mania and depression. Lancet 2:632–635
Killen JD, Fortmann SP, Schatzberg A, Hayward C, Varady A (2003) Onset of major depression during treatment for nicotine dependence. Addict Behav 28:461–470
King SL, Marks MJ, Grady SR, Caldarone BJ, Koren AO, Mukhin AG, Collins AC, Picciotto MR (2003) Conditional expression in corticothalamic efferents reveals a developmental role for nicotinic acetylcholine receptors in modulation of passive avoidance behavior. J Neurosci 23:3837–3843
Kos T, Legutko B, Danysz W, Samoriski G, Popik P (2006) Enhancement of antidepressant-like effects but not BDNF mRNA expression by the novel NMDA receptor antagonist neramexane in mice. J Pharmacol Exp Ther
Laje RP, Berman JA, Glassman AH (2001) Depression and nicotine: preclinical and clinical evidence for common mechanisms. Curr Psychiatry Rep 3:470–474
Malin DH, Lake JR, Schopen CK, Kirk JW, Sailer EE, Lawless BA, Upchurch TP, Shenoi M, Rajan N (1997) Nicotine abstinence syndrome precipitated by central but not peripheral hexamethonium. Pharmacol Biochem Behav 58:695–699
Newhouse PA, Sunderland T, Tariot PN, Blumhardt CL, Weingartner H, Mellow A, Murphy DL (1988) Intravenous nicotine in Alzheimer’s disease: a pilot study. Psychopharmacology (Berl) 95:171–175
O’Neil MF, Moore NA (2003) Animal models of depression: are there any? Hum Psychopharmacol 18:239–254
Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL (1997) Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci 17:9165–9171
Overstreet DH (1993) The Flinders sensitive line rats: a genetic animal model of depression. Neurosci Biobehav Rev 17:51–68
Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, Vincent P, Pich EM, Brulet P, Changeux JP (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374:65–67
Picciotto MR, Caldarone BJ, King SL, Zachariou V (2000) Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology 22:451–465
Picciotto MR, Brunzell DH, Caldarone BJ (2002) Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport 13:1097–1106
Pomerleau OF, Pomerleau CS (1984) Neuroregulators and the reinforcement of smoking: towards a biobehavioral explanation. Neurosci Biobehav Rev 8:503–513
Popik P, Kozela E, Krawczyk M (2003) Nicotine and nicotinic receptor antagonists potentiate the antidepressant-like effects of imipramine and citalopram. Br J Pharmacol 139:1196–1202
Porsolt RD, Bertin A, Jalfre M (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336
Salin-Pascual RJ, de la Fuente JR, Galicia-Polo L, Drucker-Colin R (1995) Effects of transderman nicotine on mood and sleep in nonsmoking major depressed patients. Psychopharmacology (Berl) 121:476–479
Semba J, Mataki C, Yamada S, Nankai M, Toru M (1998) Antidepressant-like effects of chronic nicotine on learned helplessness paradigm in rats. Biol Psychiatry 43:389–391
Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR (2002) Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry 7:525–535
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–370
Thierry B, Steru L, Simon P, Porsolt RD (1986) The tail suspension test: ethical considerations. Psychopharmacology (Berl) 90:284–285
Tizabi Y, Overstreet DH, Rezvani AH, Louis VA, Clark E Jr, Janowsky DS, Kling MA (1999) Antidepressant effects of nicotine in an animal model of depression. Psychopharmacology (Berl) 142:193–199
Wahlsten D, Metten P, Phillips TJ, Boehm SL 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC (2003) Different data from different labs: lessons from studies of gene–environment interaction. J Neurobiol 54:283–311
Acknowledgements
This work was supported by NIH grants DA13334/AA15632, MH77681, and DA00436 and the NARSAD Foster Bam Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rabenstein, R.L., Caldarone, B.J. & Picciotto, M.R. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not β2- or α7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology 189, 395–401 (2006). https://doi.org/10.1007/s00213-006-0568-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0568-z