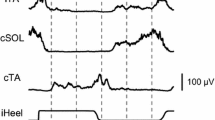
Abstract
Group I afferents in nerves innervating the lateral gastrocnemius-soleus (LG-Sol), plantaris (P1), and vastus lateralis/intermedius (VL/VI) muscles were stimulated during walking in decerebrate cats. The stimulus trains were triggered at a fixed delay following the onset of bursts in the medial gastrocnemius muscle. Stimulation of all three nerves with long stimulus trains (>600 ms) prolonged the extensor bursts and delayed the onset of flexor burst activity. LG-Sol nerve stimulation had the strongest effect; often delaying the onset of flexor burst activity until the stimulus train was ended. By contrast, flexor bursts were usually initiated before the end of the stimulus train to the P1 and VL/VI nerves. The minimum stimulus strength required to increase the cycle period was between 1.3×threshold and 1.6×threshold for all three nerves. Simultaneous stimulation of the P1 and VL/VI nerves produced a larger effect on the cycle period than stimulation of either nerve alone. The spatial summation of inputs from knee and ankle muscles suggests that the excitatory action of the group I afferents during the stance phase is distributed to all leg extensor muscles. Stimulation of the group I afferents in extensor nerves generally produced an increase in the amplitude of the heteronymous extensor EMG towards the end of the stance phase. This increase in amplitude occurred even though there were only weak monosynaptic connections between the stimulated afferents and the motoneurones that innervated these heteronymous muscles. This suggests that the excitation was produced via oligosynaptic projections onto the extensor motoneuronal pool. Stimulation with 300 ms trains during the early part of flexion resulted in abrupt termination of the swing phase and reinitiation of the stance phase of the step cycle. The swing phase resumed coincidently with the stimulus offset. Usually, stimulation of two extensor nerves at group I strengths was required to elicit this effect. We were unable to establish the relative contributions of input from the group 1a and group 1b afferents to prolonging the stance phase. However, we consider it likely that group Ib afferents contribute significantly, since their activation has been shown to prolong extensor burst activity in reduced spinal preparations. Thus, our results add support to the hypothesis that unloading of the hindlimb during late stance is a necessary condition for the initiation of the swing phase in walking animals.
Similar content being viewed by others
References
Andersson O, Grillner S (1981) Peripheral control of the cat's step cycle. I. Phase-dependent effects of ramp-movements of the hip during ‘fictive locomotion’. Acta Physiol Scand 113:89–102
Andersson O, Grillner S, Lindquist M, Zomlefer M (1978) Peripheral control of the spinal pattern generators for locomotion in the cat. Brain Res 150:625–630
Appenteng K, Prochazka A (1984) Tendon organ firing during active muscle lengthening in awake, normally behaving cats. J Physiol (Lond) 353:81–92
Conway BA, Hultborn H, Kiehn O (1987) Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res68:643–656
Coppin CML, Jack JJB, MacLennan CR (1970) A method for the selective electrical activation of tendon organ afferent fibres from the cat soleus muscle. J Physiol (Lond) 210:18–20P
Duysens J (1977) Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J Neurophysiol 40:737–751
Duysens J, Stein RB (1978) Reflexes induced by nerve stimulation in walking cats with implanted nerve cuff electrodes. Exp Brain Res 32:213–224
Duysens J, Pearson KG (1980) Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187:321–332
Eccles RM, Lundberg A (1958) Integrative patterns of Ia synaptic action on motoneurones of hip and knee muscles. J Physiol (Lond) 144:271–298
Eccles JC, Eccles RM, Lundberg A (1957a) The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol (Lond) 137:22–50
Eccles JC, Eccles RM, Lundberg A (1957b) Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol (Lond) 136:527–546
Edgley S, Jankowska E, McCrea D (1986) The heteronymous monosynaptic actions of triceps surae group Ia afferents on hip and knee extensor motoneurones in the cat. Exp Brain Res 61:443–446
Forssberg H, Grillner S (1973) The locomotion of the acute spinal cat injected with clonidine i. v. Brain Res 50:184–186
Forssberg H, Grillner S, Halbertsma J (1980) The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiol Scand 108:269–281
Gossard JP, Brownstone RM, Barajon I, Hultborn H (1994) Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98:213–228
Grillner S (1981) Control of locomotion in bipeds, tetrapods and fish. In: Brooks VB (ed) Handbook of physiology, sect 1, The nervous system, part II, motor control. American Physiology Society, Waverly Press, Bethesda, Md, pp 1179–1236
Grillner S, Rossignol S (1978) On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res 146:269–277
Guertin P, Angel MJ, Perreault MC, Carr PA, McCrea DA (1993) Ankle extensor group I afferents excite extensors throughout the hindlimb during MLR evoked fictive locomotion. Soc Neurosci Abstr 19:142
Hiebert GW, Whelan PJ, Prochazka A, Pearson KG (1995) Suppression of the corrective response to loss of ground support by stimulation of extensor group I afferents. J Neurophysiol (in press)
Jack JJB (1978) Some methods for selective activation of muscle afferent fibres. In: Porter R (ed) Studies in neurophysiology. Cambridge University Press, Cambridge, UK, pp 155–176
Lundberg A (1969) Reflex control of stepping. The Nansen memorial lecture to Norwegian Academy of Sciences. Universitetsforlaget, Oslo
Lundberg A (1980) Half-centres revisited. In: Szentagothai J, Palkovits M, Jamori J (eds) Regulatory functions of the CNS. Motion and organization principles. Pergamon Press, London, pp 155–167
Orsal D, Cabelguen JM, Perret C (1990) Interlimb coordination during fictive locomotion in the thalamic cat. Exp Brain Res 82:536–546
Pearson KG, Collins DF (1993) Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol 70:1009–1017
Pearson KG, Ramirez JM, Jiang W (1992) Entrainment of the locomotor rhythm by group Ib afferents from ankle extensor muscles in spinal cats. Exp Brain Res 90:557–566
Rossignol S, Lund JP, Drew T (1988) The role of sensory inputs in regulating patterns of rhythmical movements in higher vertebrates. In: Cohen A, Rossignol S, Grillner S. (eds) Neural control of rhythmical movements in vertebrates. Wiley, New York, pp 201–283
Sherrington CS (1947) The integrative action of the nervous system. Cambridge University Press, Cambridge, UK
Shik ML, Severin FV, Orlovsky GN (1966) Control of walking and running by means of electrical stimulation of the midbrain. Biophysics 11:756–765
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Whelan, P.J., Hiebert, G.W. & Pearson, K.G. Stimulation of the group I extensor afferents prolongs the stance phase in walking cats. Exp Brain Res 103, 20–30 (1995). https://doi.org/10.1007/BF00241961
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00241961